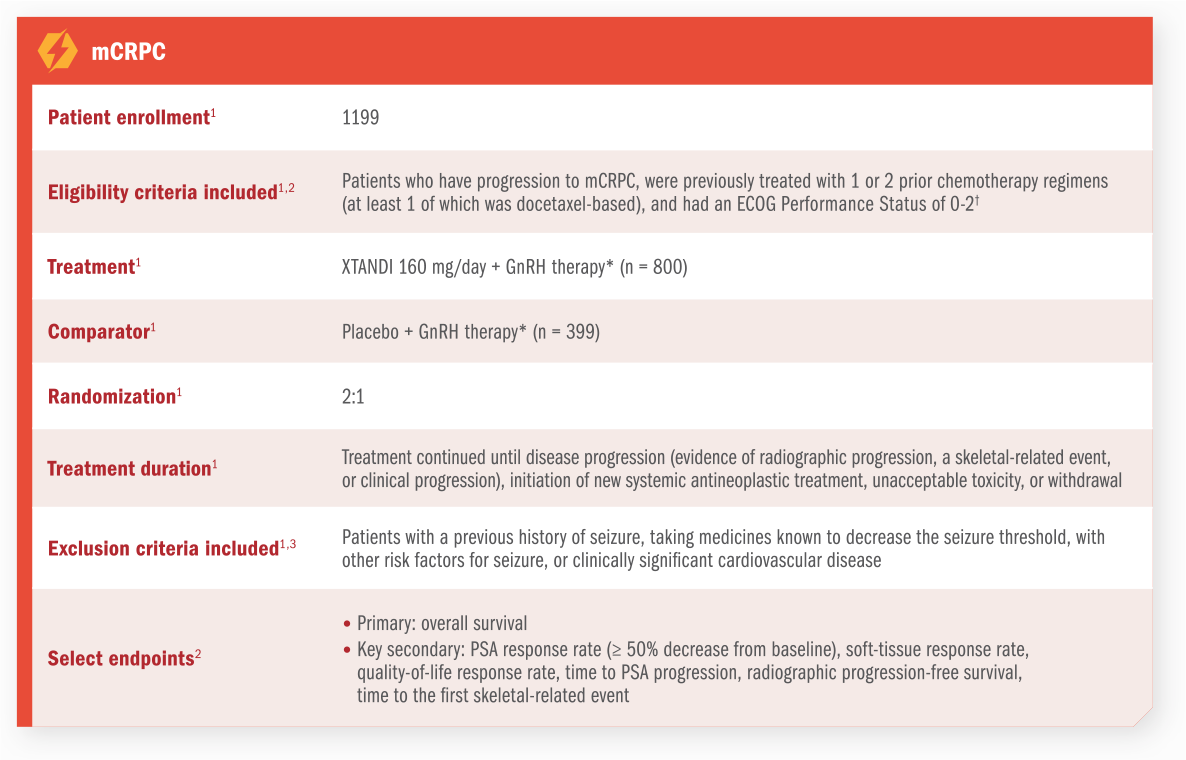
Understanding CRPC
CRPC is defined as1:
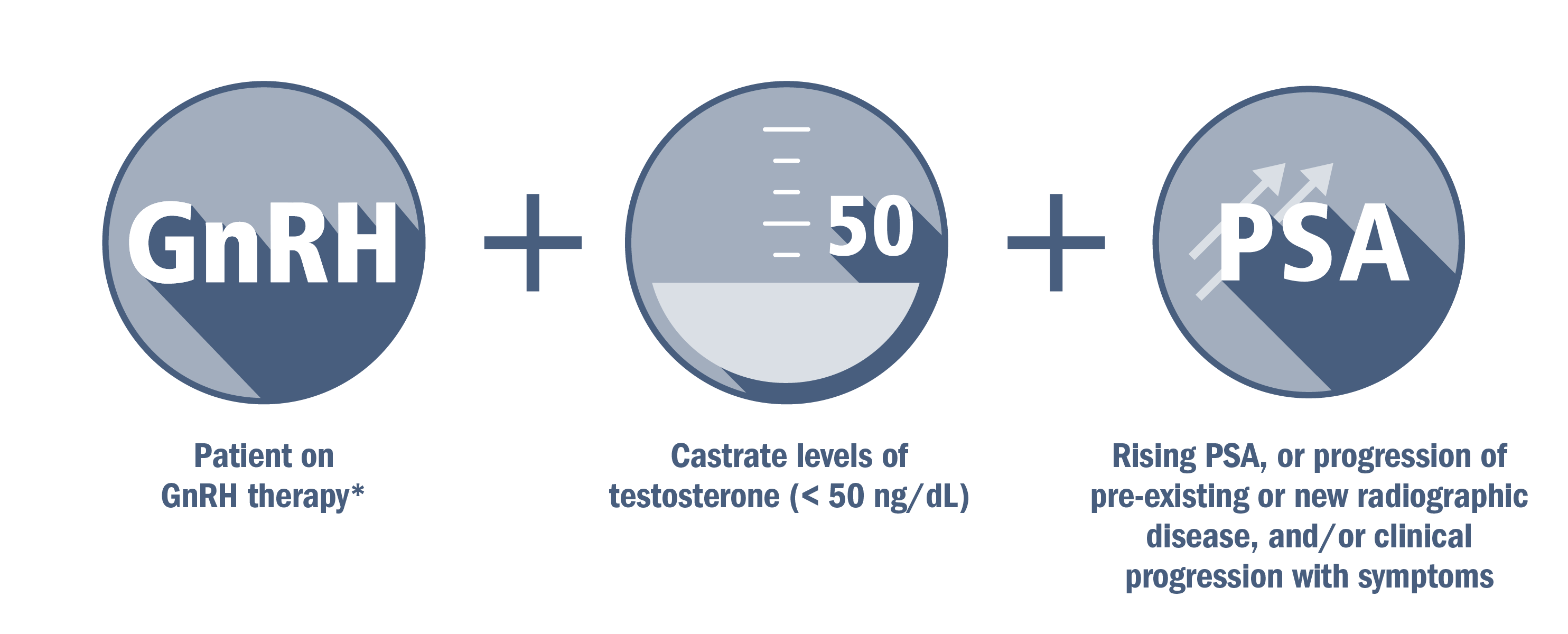
CRPC can be nonmetastatic (no radiographic evidence of metastasis) or metastatic (evidence of metastatic disease).
*Or after surgical castration.1
One study estimated that patients treated with GnRH therapy* stop responding within 1-3 years2
The median time to progression† on GnRH therapy* (also known as androgen deprivation therapy) was assessed in a retrospective analysis of 553 patients initiating GnRH therapy* (+/- anti-androgen) with metastatic (49%) or nonmetastatic (51%) castration-sensitive prostate cancer. Median follow-up was 5.1 years2
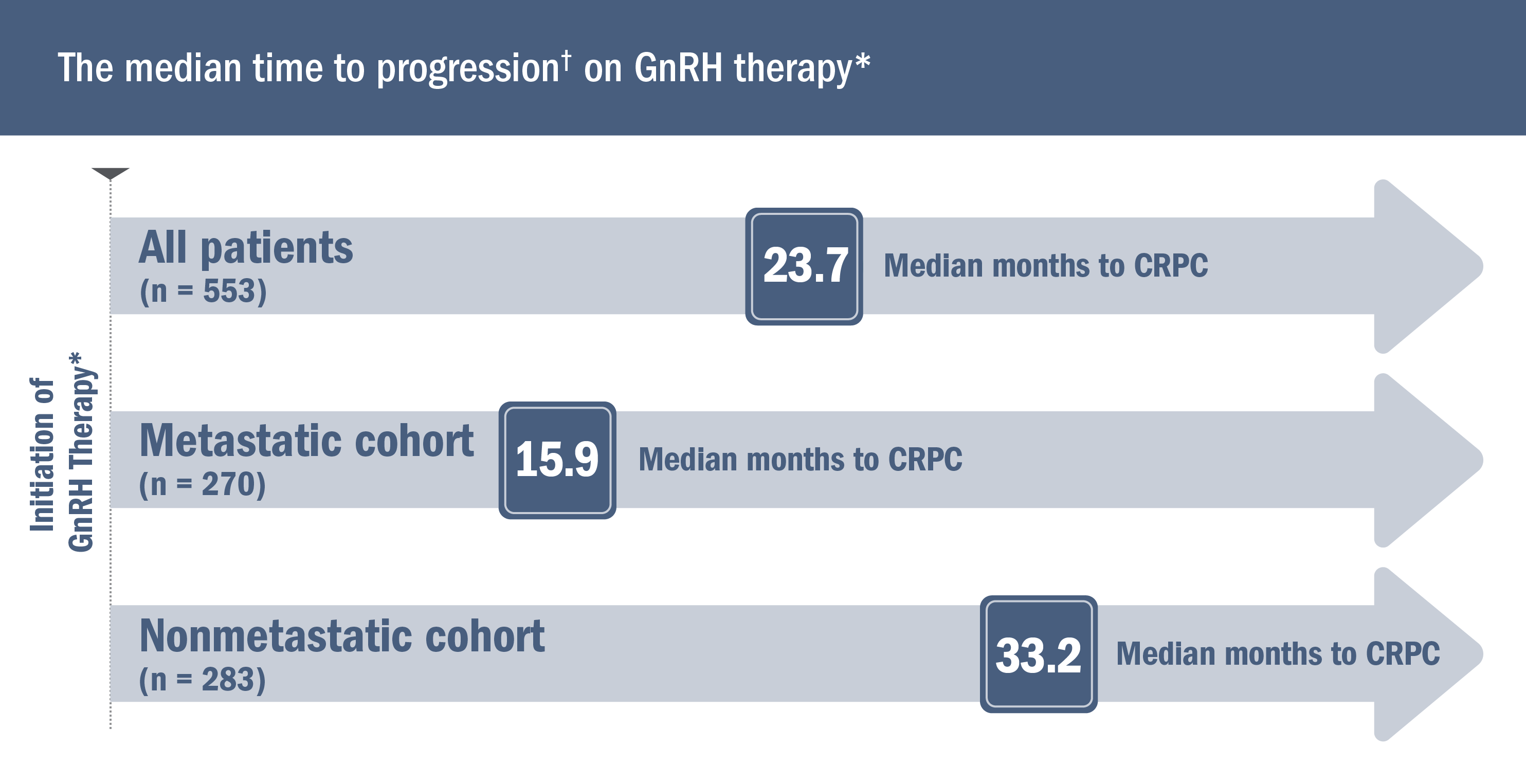
*Or after surgical castration.1
†Defined as the duration of time from GnRH therapy* initiation to the date of progression (2 rises in PSA above a nadir value while receiving GnRH therapy*). The date of progression was defined as the date of the first rise.2
Patients who progress on GnRH therapy* may have asymptomatic mCRPC3
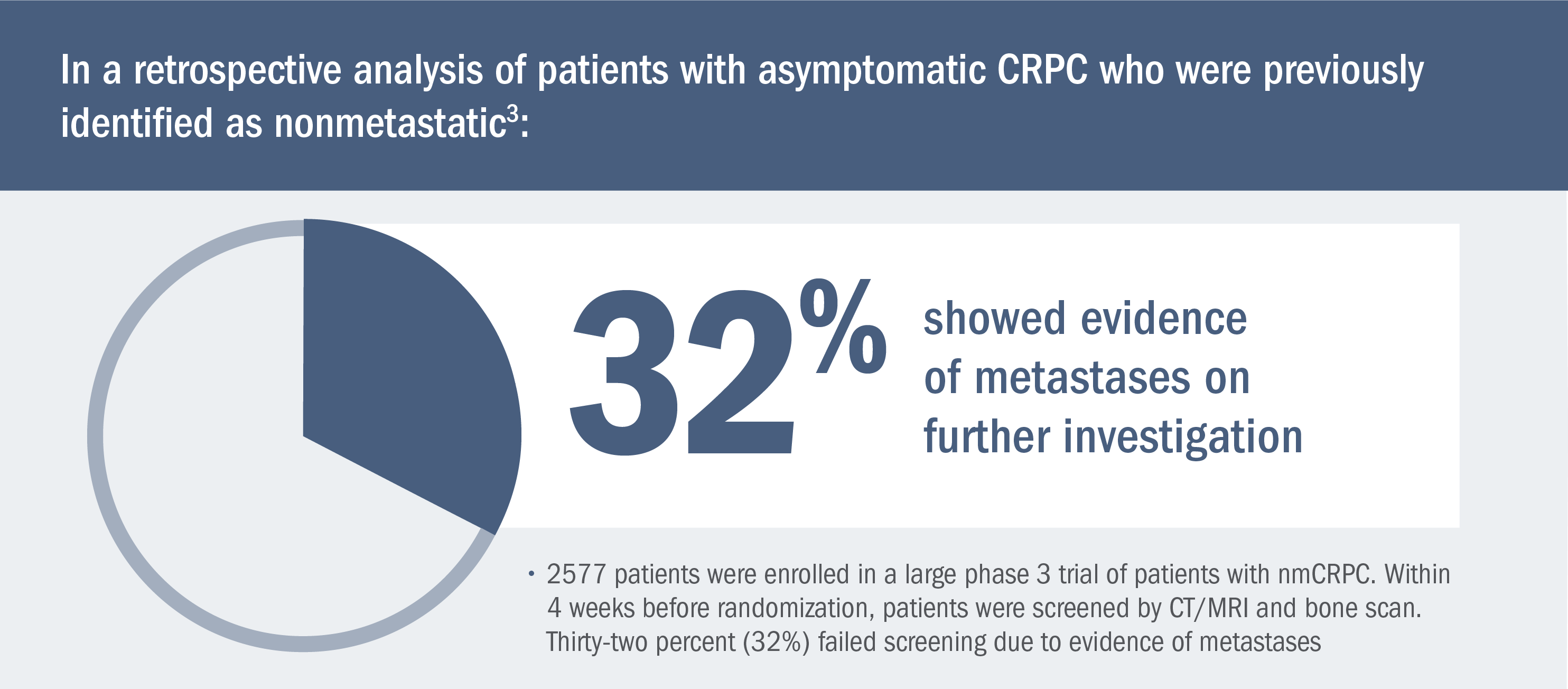
*Or after surgical castration.1
“The unexpectedly high rate of metastatic disease in this trial suggests that a high proportion of men thought to have nonmetastatic CRPC may have had asymptomatic metastasis.”
—Yu EY et al. (2012)3
Retrospective analysis suggests an association between metastatic burden and clinical outcomes4
In a retrospective analysis of bone metastases among patients with mCRPC, those with fewer bone lesions at baseline experienced better overall survival and progression-free survival4:
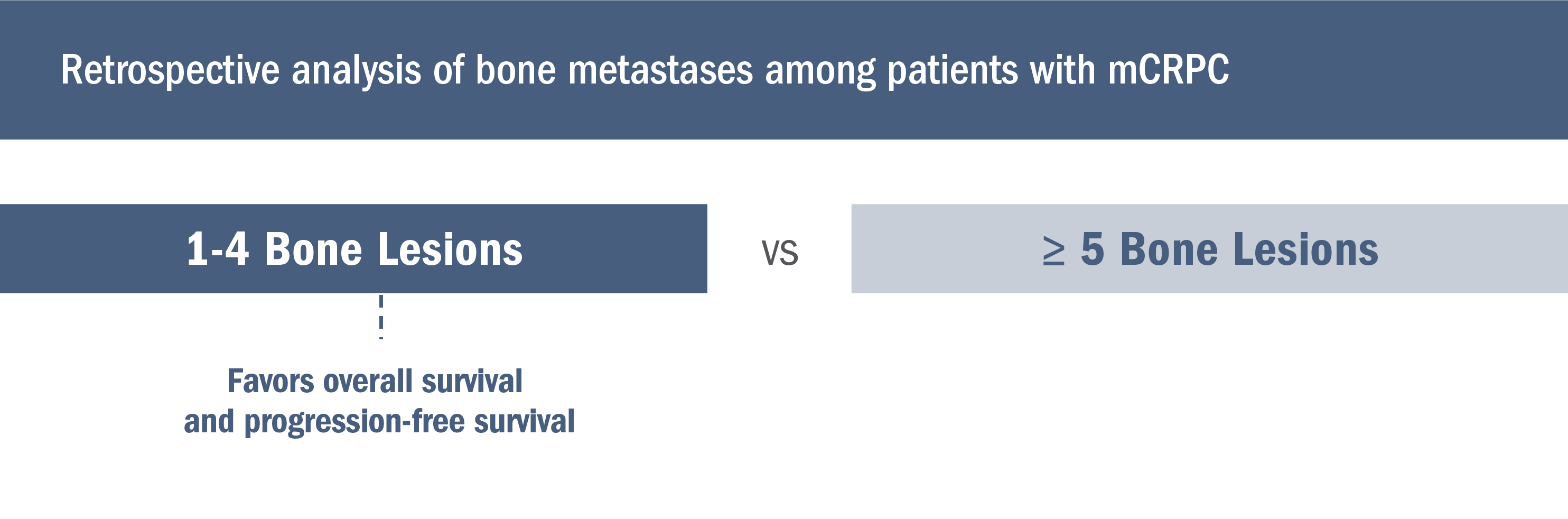
- From a retrospective analysis of 561 patients with confirmed bone metastases from a randomized phase 3 trial, bone metastases at trial entry were confirmed by bone scintigraphy supplemented by CT and/or MRI (where metastases were equivocal). The trial ended early due to a lack of efficacy, which allowed the authors to combine both cohorts and correlate the number of bone metastases present at baseline with the natural history of mCRPC4
Meta-analysis evaluating the impact of site of metastases on overall survival in men with CRPC5
This meta-analysis of nine phase 3 studies included 8736 men with CRPC who received docetaxel chemotherapy and was used to evaluate the impact of site of metastases on overall survival5
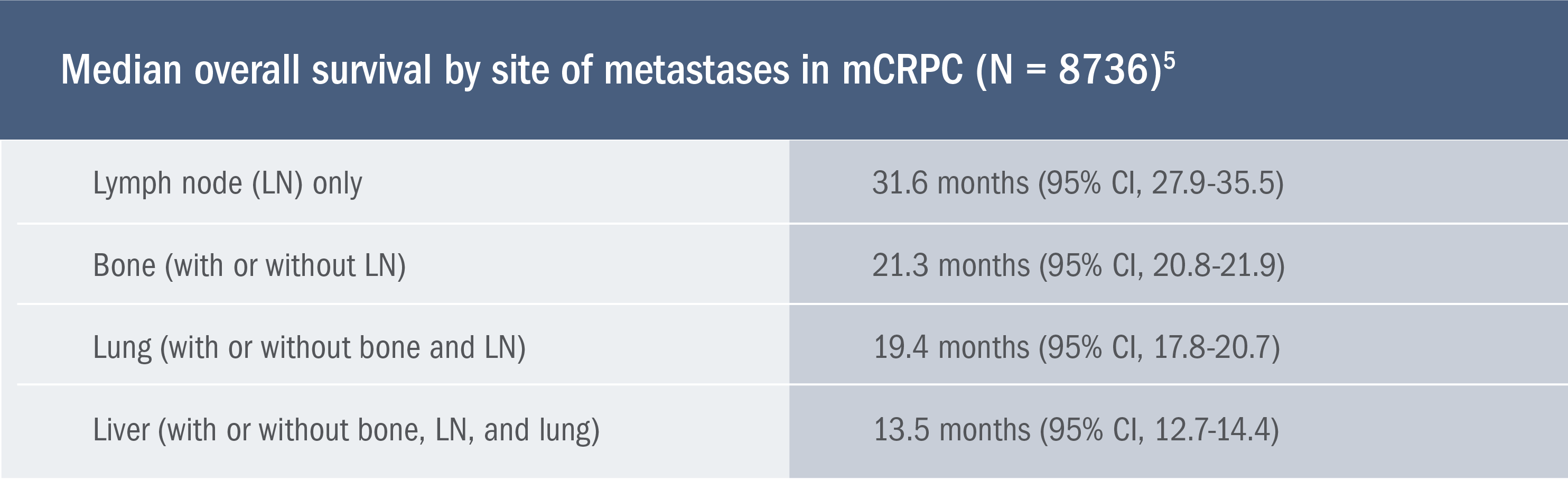
- Limitations of this retrospective analysis include the inability to account for all known prognostic factors across trials. In addition, neither imaging nor imaging reports were centrally reviewed, which prevented an assessment of the impact of metastatic burden and number of metastases5
Early detection of advancing disease may help inform clinical decision-making6,7
Recommendations for the early identification of metastases from the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence (RADAR) Group6,7
The RADAR I Group initially convened to provide recommendations for the early identification of prostate cancer metastases using traditional imaging modalities.
The RADAR III Group convened to evaluate the use of next-generation imaging (NGI) modalities and review the rationale behind choosing certain scans, frequency of scanning, interpreting results, and subsequent clinical usefulness.
RADAR III acknowledges the limitations of making recommendations when level 1 evidence-based data are not yet available.
The RADAR VI Group convened to develop pathways and recommendations regarding how new targeted precision imaging (TPI) modalities could be used, given recent approvals of radiotracers.
CRPC scanning recommendations: RADAR7

Understanding the mechanism of disease (MOD) and implications in nmCSPC with high-risk BCR, mCSPC, and CRPC
Mechanism of Disease
GnRH therapy and prostate cancer cell adaptation
In response to GnRH therapy, prostate cancer cells may adapt so that androgen receptor signaling continues to drive cell growth8-12

See how XTANDI may be able to help patients with CRPC
ADT, androgen deprivation therapy; BCR, biochemical recurrence; CT, computed tomography; CRPC, castration-resistant prostate cancer; FDG, fluorodeoxyglucose; GnRH, gonadotropin-releasing hormone; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; MRI, magnetic resonance imaging; nmCRPC, nonmetastatic castration-resistant prostate cancer; nmCSPC, nonmetastatic castration-sensitive prostate cancer; PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen.
References: 1. Lowrance W, Dreicer R, Jarrard DF, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. 2023;209(6):1082-1090. doi:10.1097/JU.0000000000003452. 2. Ross RW, Xie W, Regan MM, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008:112(6):1247-1253. doi:10.1002/cncr.23304. 3. Yu EY, Miller K, Nelson J, et al. Detection of previously unidentified metastatic disease as a leading cause of screening failure in a Phase III trial of zibotentan versus placebo in patients with nonmetastatic, castration resistant prostate cancer. J Urol. 2012;188(1):103-109. doi:10.1016/j.juro.2012.03.008. 4. Tait C, Moore D, Hodgson C, et al. Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int. 2014;114(6b):E70-73. doi:10.1111/bju.12717. 5. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652-1659. doi:10.1200/JCO.2015.65.7270. 6. Crawford ED, Koo PJ, Shore N, et al. A clinician’s guide to next generation imaging in patients with advanced prostate cancer (RADAR III). J Urol. 2019;201(4):682-692. doi:10.1016/j.juro.2018.05.164. 7. Crawford ED, Albala DM, Harris RG, et al. A clinician's guide to targeted precision imaging in patients with prostate cancer (RADAR VI) [published January 2023]. JU Open Plus. 2023. https://journals.lww.com/juop/fulltext/2023/01000/a_clinician_s_guide_to_targeted_precision_imaging.3.aspx. 8. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33-39. doi:10.1038/nm972. 9. Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217-227. doi:10.1016/S0002-9440(10)63112-4. 10. Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912-2918. doi:10.1158/0008-5472.CAN-08-3667. 11. Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393-1398. doi:10.1056/NEJM199505253322101. 12. Linja MJ, Savinainen KJ, Saramäki OR, Tammela TLJ, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550-3555. 13. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787-790. doi:10.1126/science.1168175. 14. Bubendorf L, Kononen J, Koivisto P, et al. Survey of gene amplifications during prostate cancer progression by high-throughput fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59(4):803-806. Erratum in: Cancer Res. 1999;59(6):1388. 15. Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314-319. 16. Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72(9):2176-2182. doi:10.1158/0008-5472.CAN-11-3980. 17. Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703-706. Erratum in: Nat Med. 2000;6(8):939. doi:10.1038/76287. 18. Veldscholte J, Ris-Stalpers C, Kuiper GG, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534-540. doi:10.1016/s0006-291x(05)80067-1. 19. Libertini SJ, Tepper CG, Rodriguez V, Asmuth DM, Kung HJ, Mudryj M. Evidence for calpain-mediated androgen receptor cleavage as a mechanism for androgen independence. Cancer Res. 2007;67(19):9001-9005. doi:10.1158/0008-5472.CAN-07-1072. 20. Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16-22. doi:10.1158/0008-5472.CAN-08-2764. 21. Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469-5477. doi:10.1158/0008-5472.CAN-08-0594.