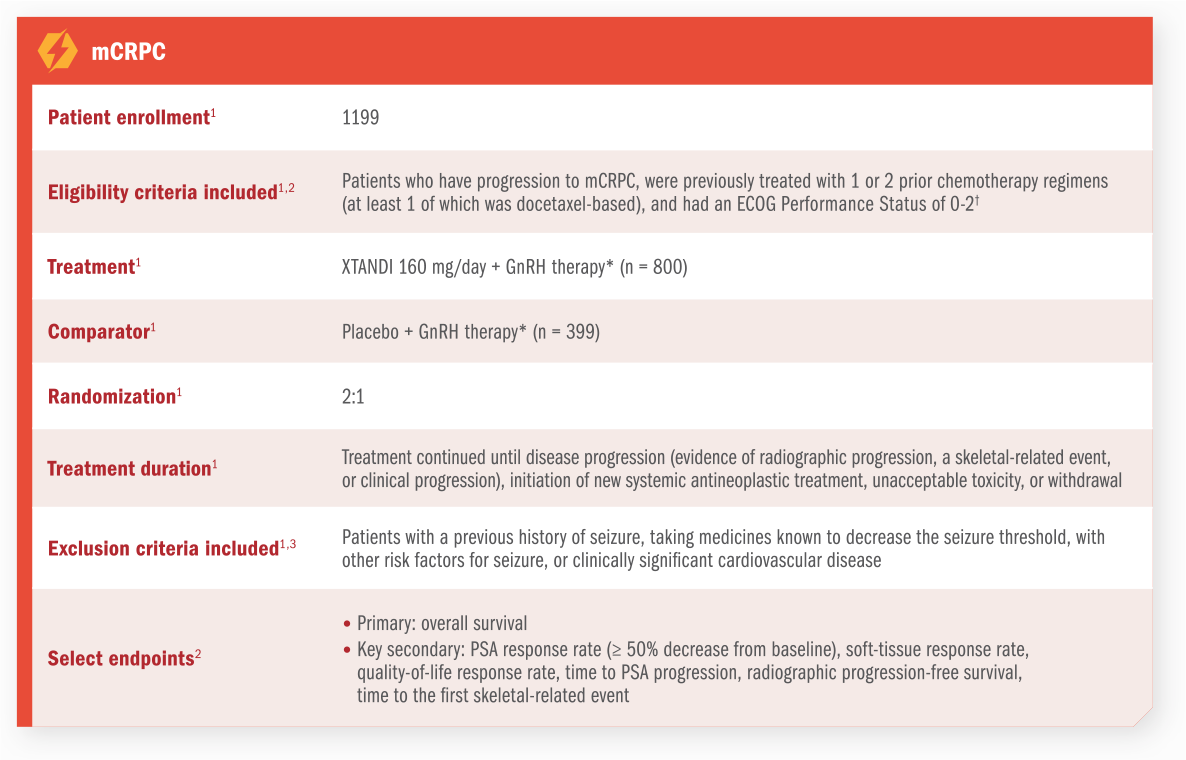
ARCHES Trial
XTANDI + GnRH therapy* significantly extended radiographic progression-free survival†1
ARCHES assessed the efficacy and safety of XTANDI + GnRH therapy* vs placebo + GnRH therapy* in patients with mCSPC.1
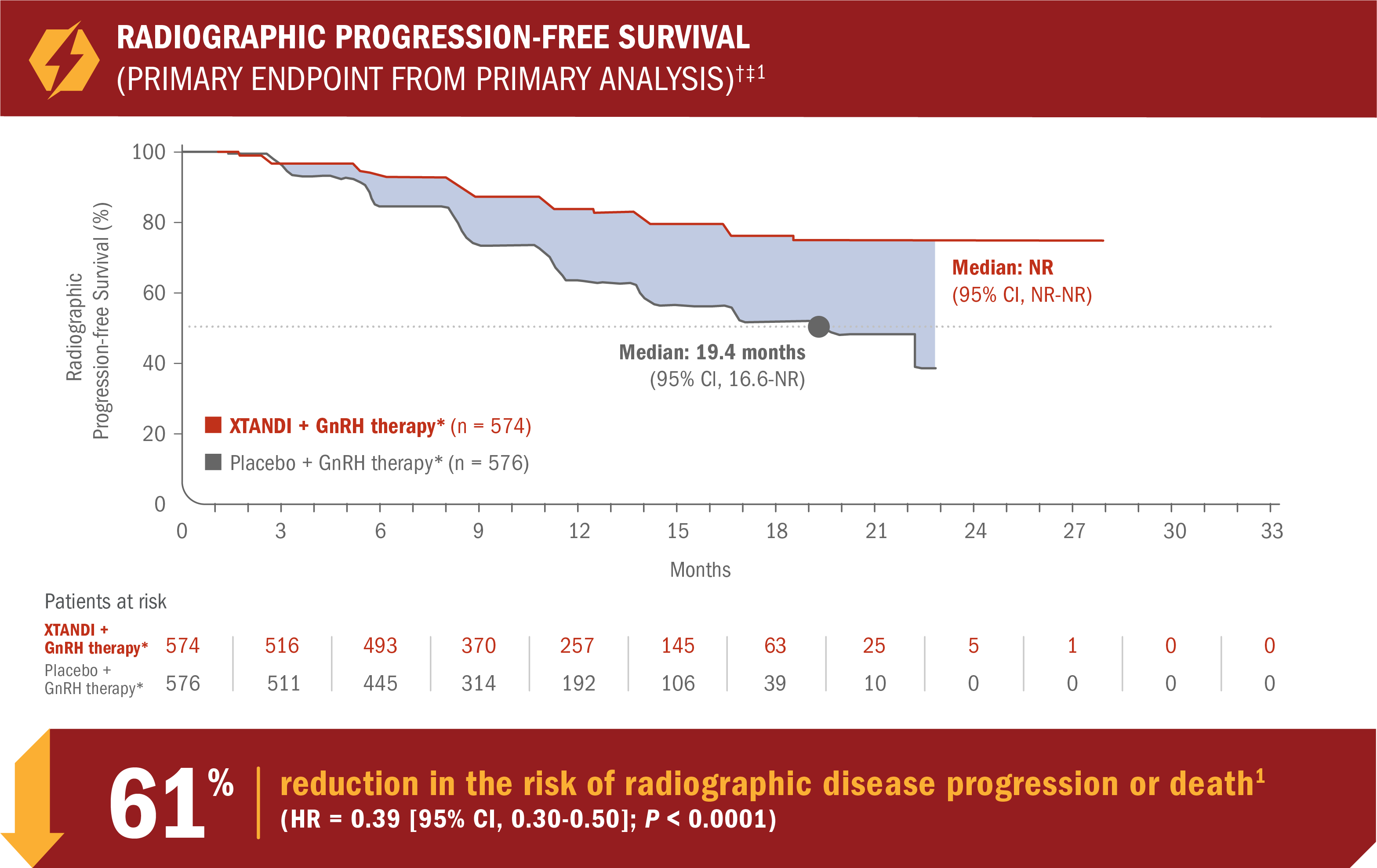
- Number of events: 89 (15.5%) with XTANDI + GnRH therapy* vs 198 (34.4%) with placebo + GnRH therapy*1
- Consistent radiographic progression-free survival results were observed in patients with low and high§ volume of disease and patients with and without prior docetaxel therapy1
See mCSPC (ARCHES) adverse reactions
*Or after bilateral orchiectomy.1
†Radiographic progression-free survival was defined as the time from randomization to radiographic disease progression at any time or death within 24 weeks after study drug discontinuation.1
‡At the time of analysis, the median follow-up was 14.4 months.2
§Defined as metastases involving the viscera or, in the absence of visceral lesions, ≥ 4 bone lesions, ≥ 1 of which must be in a bony structure beyond the vertebral column and pelvic bone.1
ARCHES assessed the efficacy and safety of XTANDI + GnRH therapy* vs placebo + GnRH therapy* in patients with mCSPC1
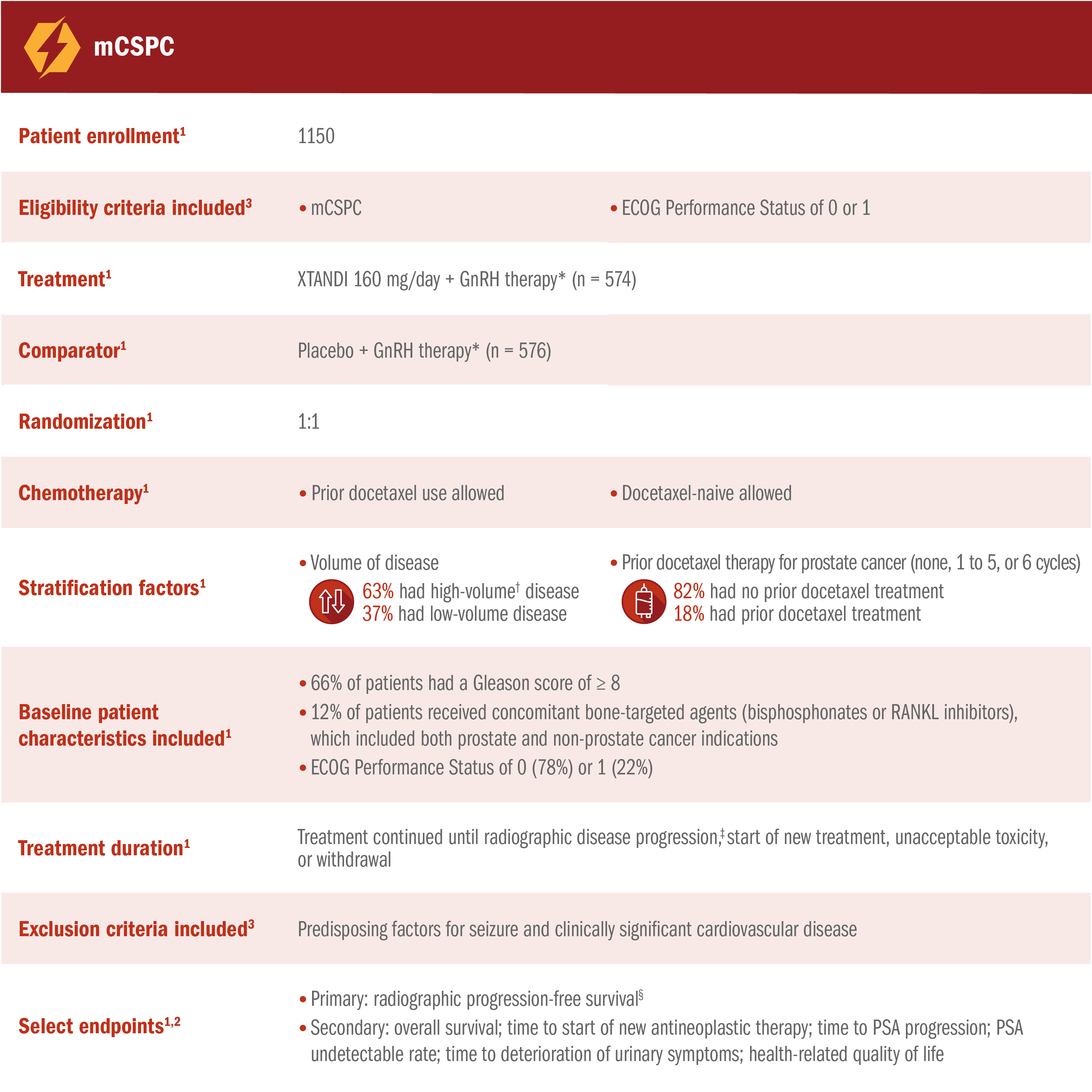
*Or after bilateral orchiectomy.1
†Defined as metastases involving the viscera or, in the absence of visceral lesions, ≥ 4 bone lesions, ≥ 1 of which must be in a bony structure beyond the vertebral column and pelvic bone.1
‡Radiographic disease progression was defined by identification of ≥ 2 new bone lesions on a bone scan with confirmation (PCWG2 criteria) and/or progression in soft-tissue disease.1
§Radiographic progression-free survival was defined as the time from randomization to radiographic disease progression at any time or death within 24 weeks after study drug discontinuation.1
XTANDI + GnRH therapy* significantly extended overall survival1
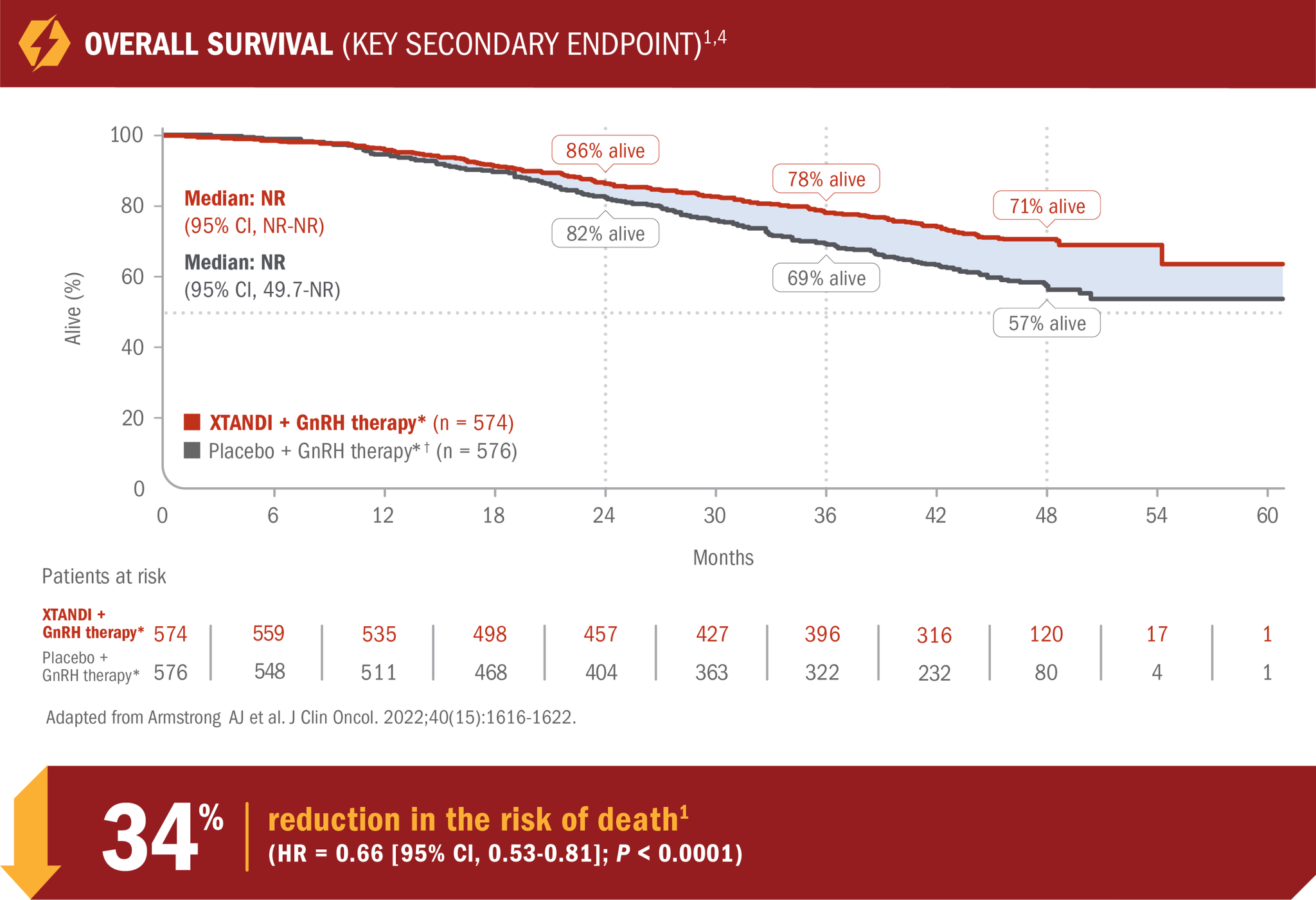
- Number of events: 154 (26.8%) with XTANDI + GnRH therapy* vs 202 (35.1%) with placebo + GnRH therapy*1
- At 24, 36, and 48 months, 86%, 78%, and 71% of patients randomized to XTANDI + GnRH therapy* were alive according to Kaplan-Meier calculations, respectively, compared with 82%, 69%, and 57% of patients randomized to placebo + GnRH therapy.*† This was not a prespecified analysis and is not in the US Full Prescribing Information for XTANDI4
*Or after bilateral orchiectomy.1
†In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy* remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy.* The median time to crossover was 21.5 months.4
The information below is not included in the US Full Prescribing Information for XTANDI. Subgroups were prespecified but not alpha protected and should be considered exploratory analyses. These subgroup analyses are presented for descriptive purposes and cannot be interpreted as a demonstration of efficacy in any particular subgroup.
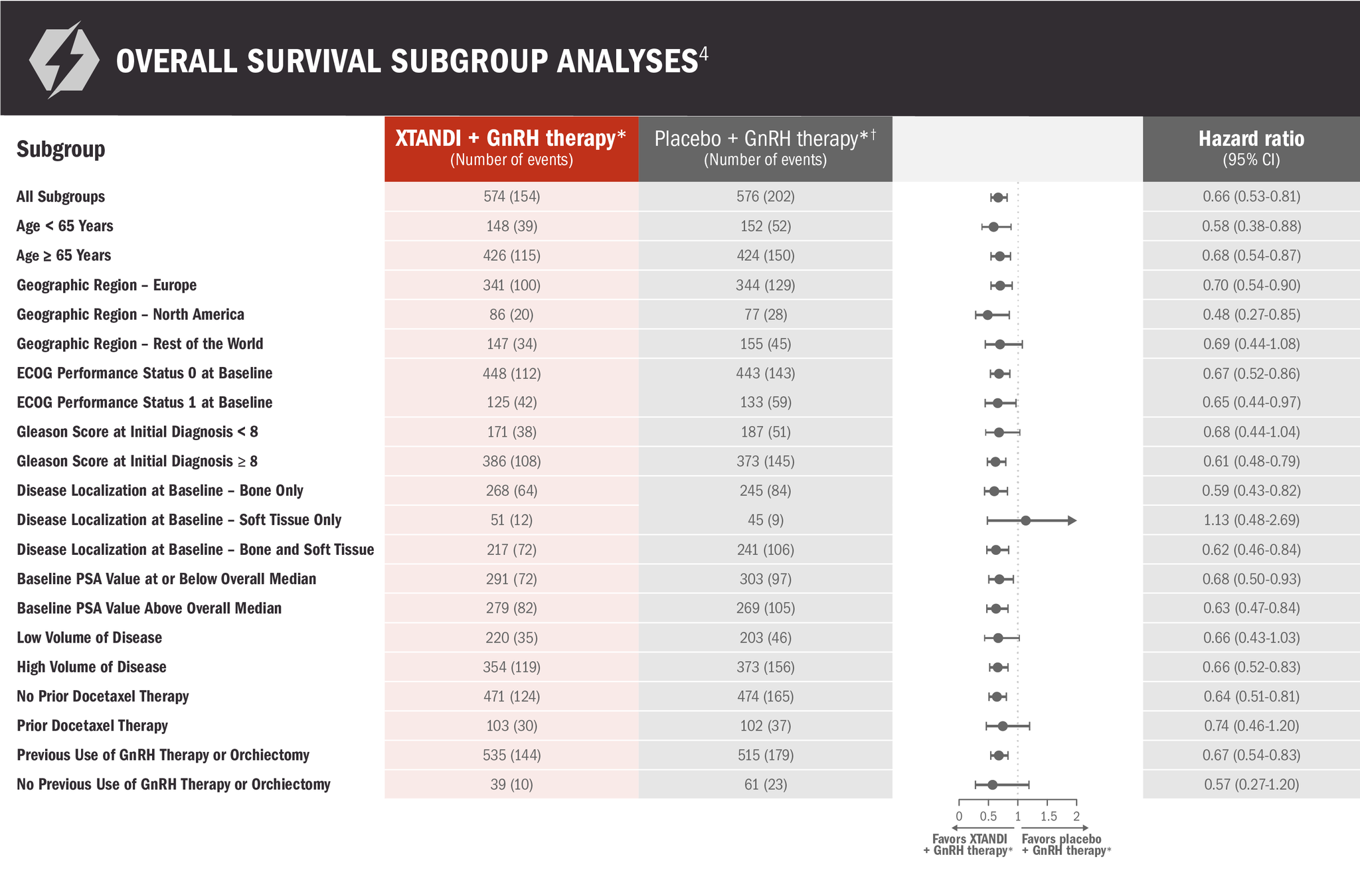
*Or after bilateral orchiectomy.1
†In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy* remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy.* The median time to crossover was 21.5 months.4
The information below is not included in the USPI for XTANDI. There was no assessment of statistical significance for this post-hoc analysis; it was not alpha-protected and should be understood as descriptive information.
A post-hoc analysis of overall survival in patients with mCSPC was performed at 5 years6
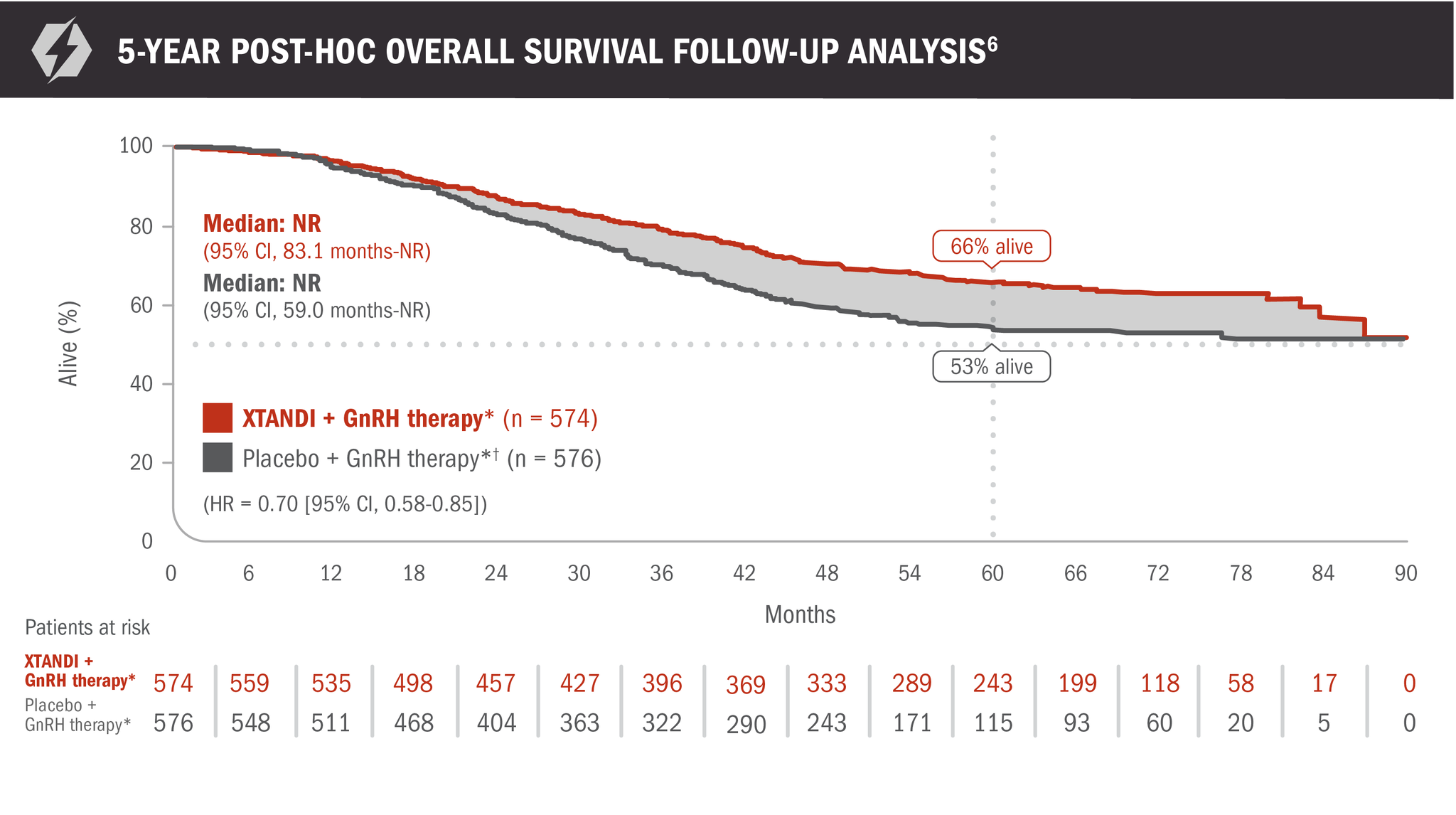
- Number of events: 191 (33.3%) with XTANDI + GnRH therapy* and 223 (38.7%) with placebo + GnRH therapy*6
- At 60 months, 66% of patients randomized to XTANDI + GnRH therapy* and 53% of patients randomized to placebo + GnRH therapy* were alive according to Kaplan-Meier calculations6
*Or after bilateral orchiectomy.1
†In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy* remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy.* The median time to crossover was 21.5 months.4
XTANDI + GnRH therapy* significantly delayed time to start of new antineoplastic therapy† in patients with mCSPC1
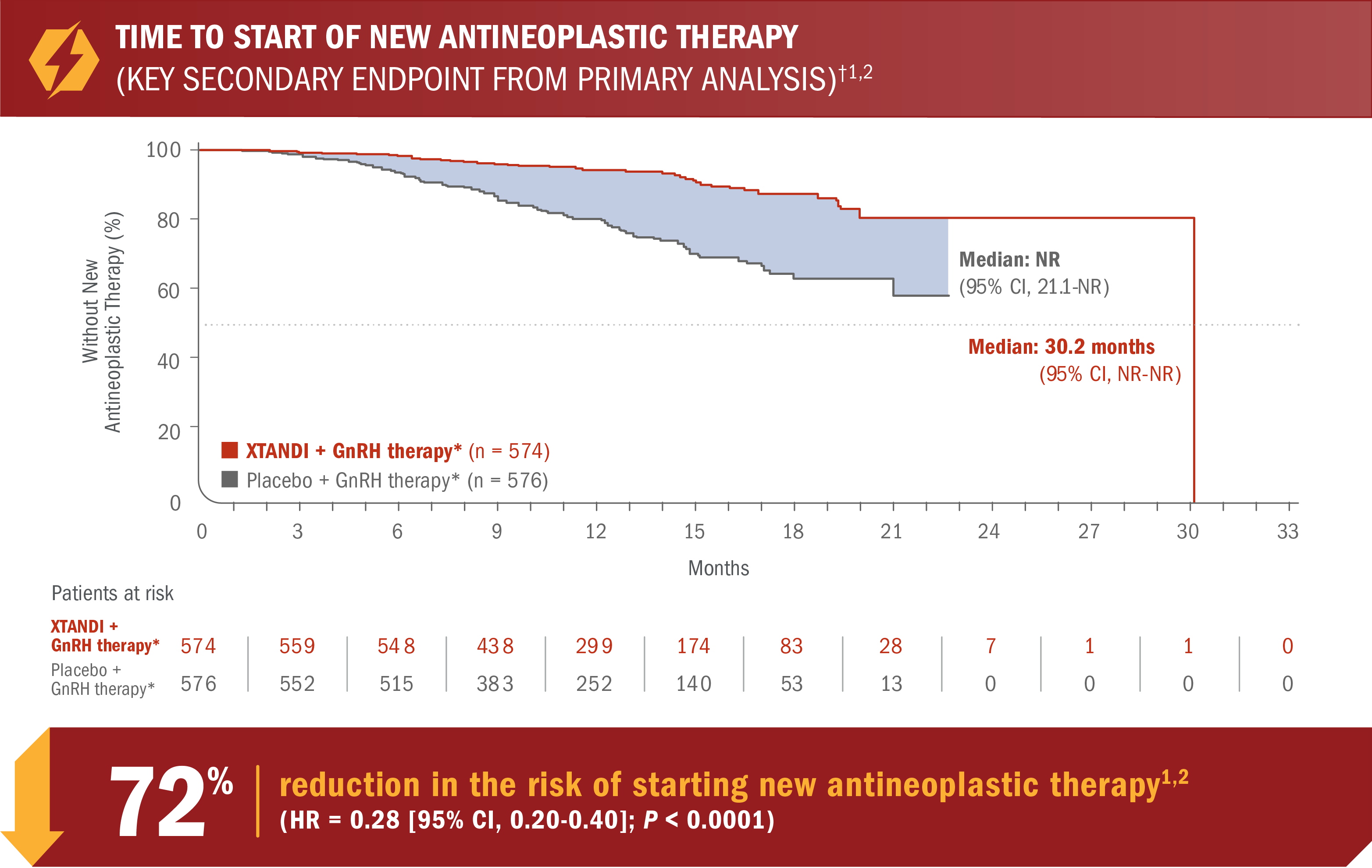
- Number of events: 46 (8.0%) with XTANDI + GnRH therapy* vs 133 (23.1%) with placebo + GnRH therapy*5
*Or after bilateral orchiectomy.1
†Time to start of new antineoplastic therapy was defined as the time from randomization to the initiation of antineoplastic therapy subsequent to the study treatments.6
An updated analysis of time to start of new antineoplastic therapy was performed at the time of final overall survival analysis4
The information below is not included in the US Full Prescribing Information for XTANDI. An update to the analysis of time to start of new antineoplastic therapy was performed at the time of the final overall survival analysis. There was no assessment of statistical significance for this updated analysis, and it should be understood as descriptive information.
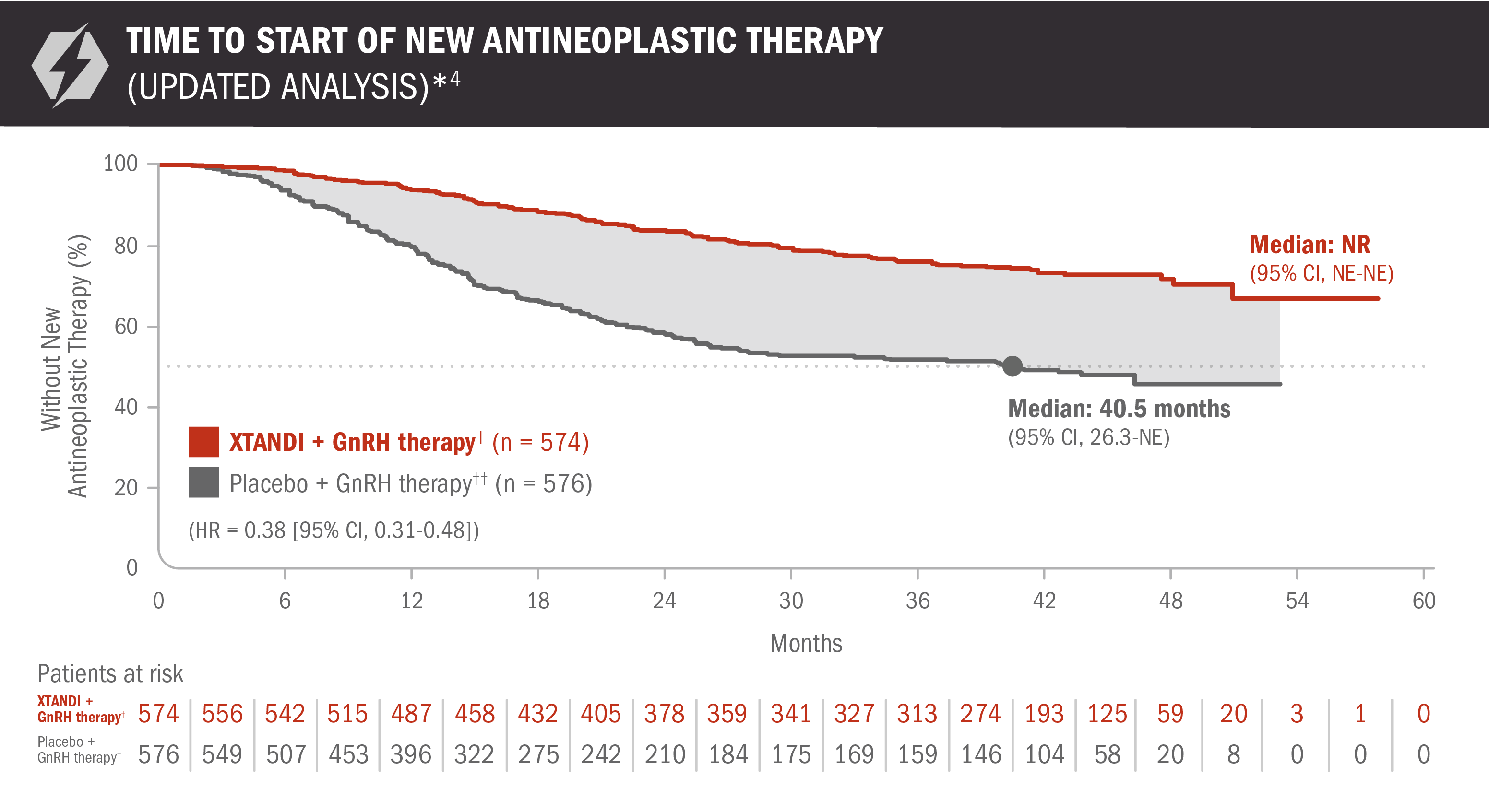
- Number of events: 131 (22.82%) with XTANDI + GnRH therapy† and 236 (40.97%) with placebo + GnRH therapy†6
Time to start of new antineoplastic therapy was defined as the time from randomization to the initiation of antineoplastic therapy subsequent to the study treatments.6
*For the analysis of time to start of new antineoplastic therapy, crossover to XTANDI after the primary radiographic progression-free survival analysis was not counted as a subsequent therapy.7
†Or after bilateral orchiectomy.1
‡In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy† remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy.† The median time to crossover was 21.5 months.4
Time to PSA progression was also evaluated in patients with mCSPC2
The information below is not included in the US Full Prescribing Information for XTANDI. PSA is not a reliable surrogate for overall survival. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.
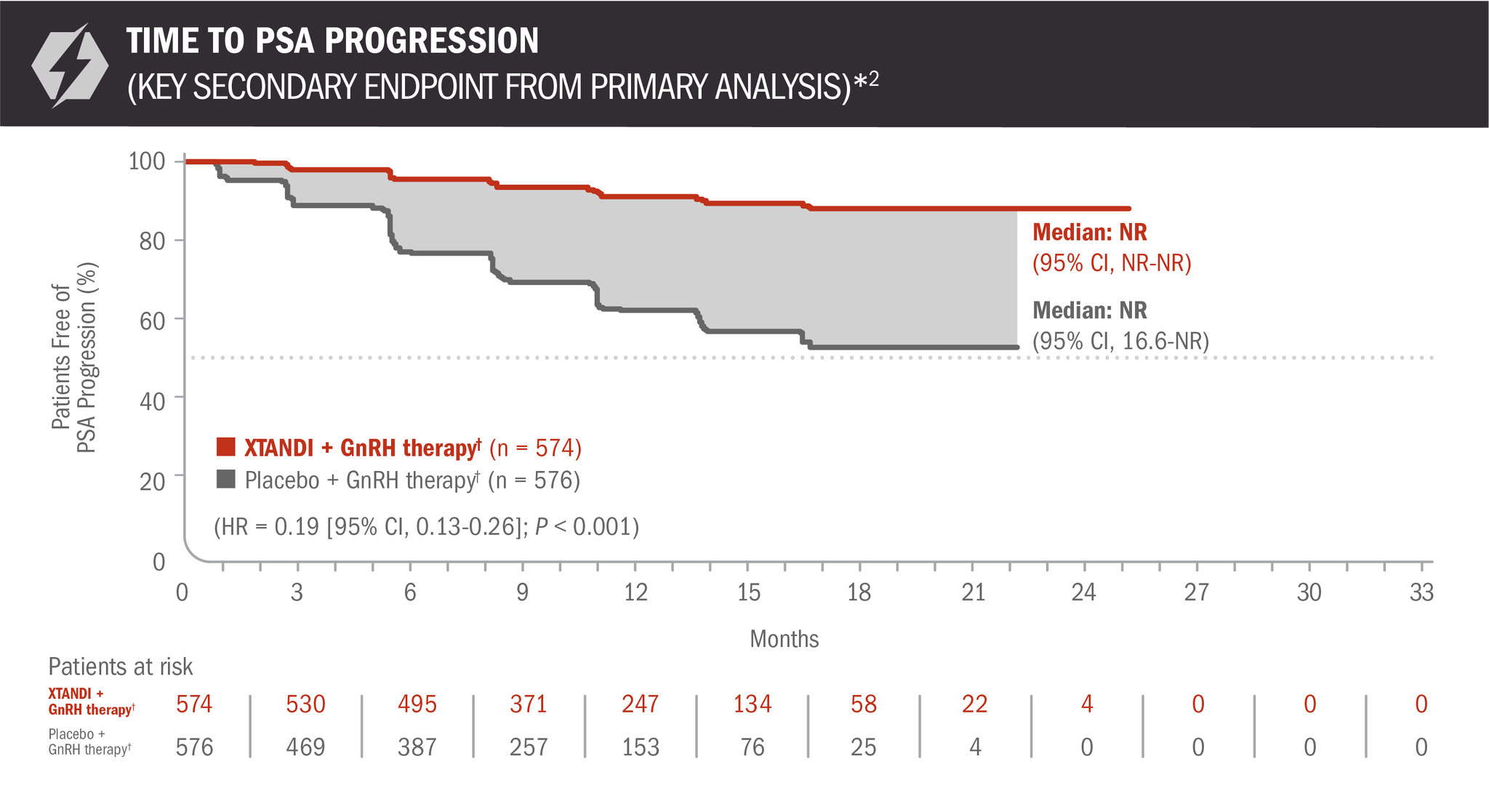
- Number of events: 45 (7.84%) with XTANDI + GnRH therapy† and 189 (32.81%) with placebo + GnRH therapy†6
*Time to PSA progression was defined as the time from randomization to PSA progression, which was a ≥ 25% increase and an absolute increase of ≥ 2 μg/L (≥ 2 ng/mL) above the nadir (ie, the lowest PSA value observed post baseline or at baseline), which was confirmed by a second consecutive value at least 3 weeks later.3
†Or after bilateral orchiectomy.1
The information below is not included in the US Full Prescribing Information for XTANDI. An update to the analysis of time to PSA progression was performed at the time of the final overall survival analysis. There was no assessment of statistical significance for this post-hoc analysis, and it should be understood as descriptive information.
A post-hoc analysis of time to PSA progression was performed at the time of final overall survival analysis4,7
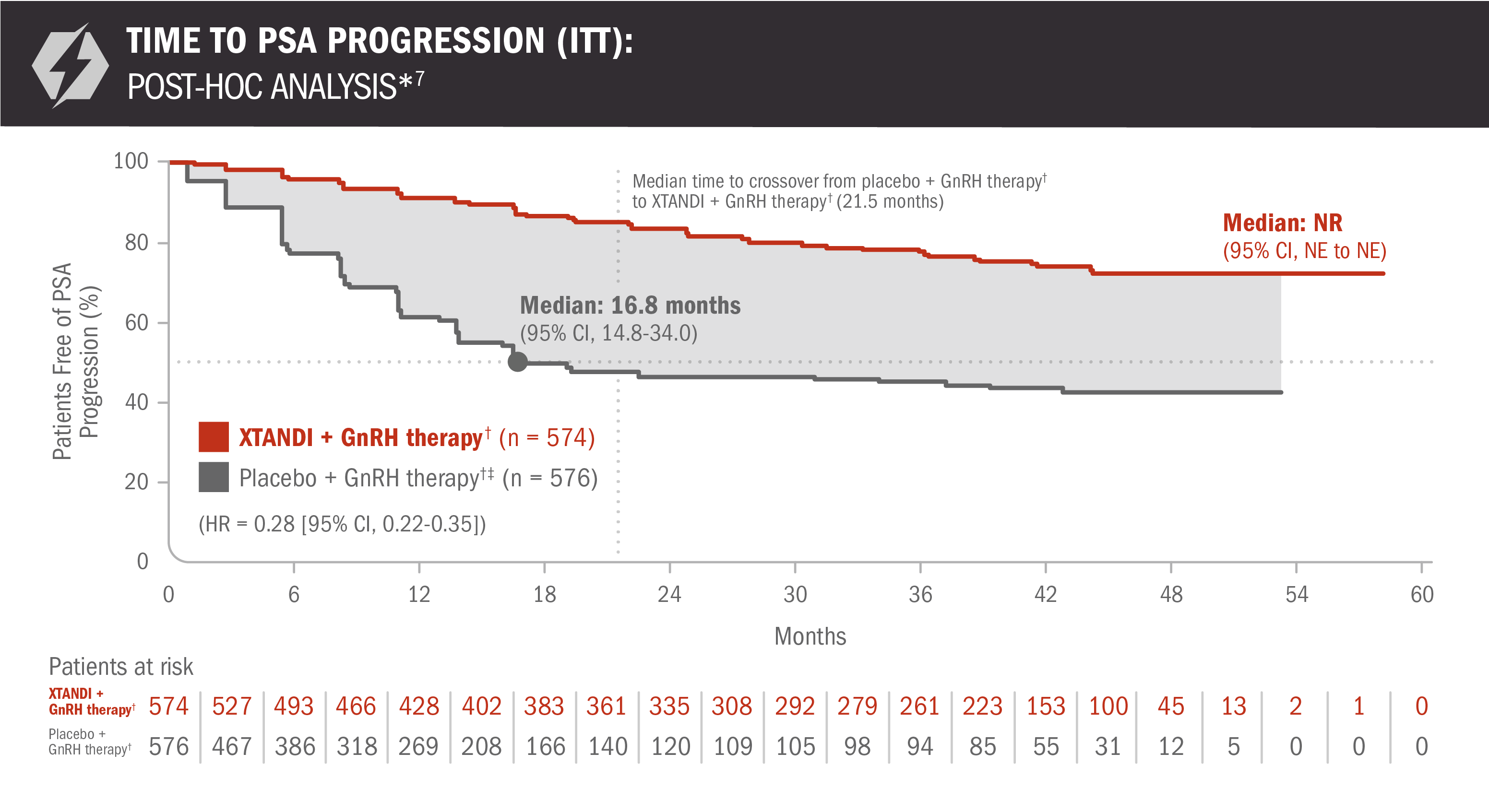
- Number of events: 117 (20.4%) with XTANDI + GnRH therapy† and 259 (45.0%) with placebo + GnRH therapy†6
*Time to PSA progression was defined as the time from randomization to PSA progression, which was a ≥ 25% increase and an absolute increase of ≥ 2 μg/L (≥ 2 ng/mL) above the nadir (ie, the lowest PSA value observed post baseline or at baseline), which was confirmed by a second consecutive value at least 3 weeks later.3
†Or after bilateral orchiectomy.1
‡In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy† remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy.† The median time to crossover was 21.5 months.4
PSA undetectable rate and time to deterioration of urinary symptoms were also evaluated in patients with mCSPC1,6
PSA is not a reliable surrogate for overall survival. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.
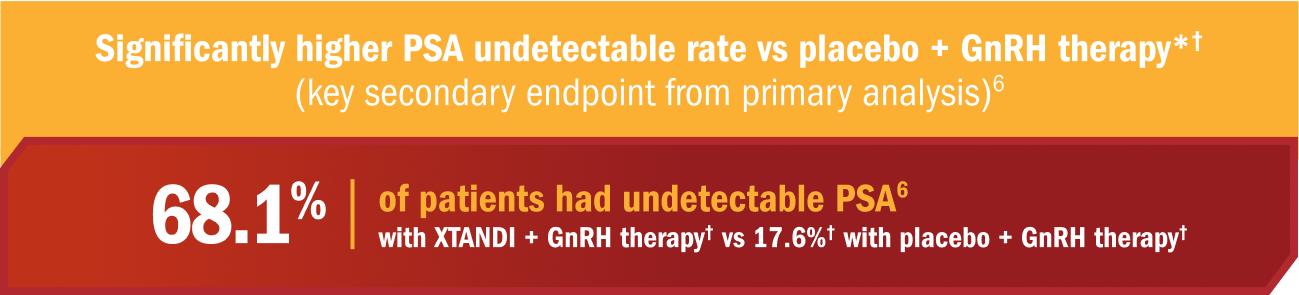
- XTANDI in addition to androgen deprivation therapy reduced PSA levels to undetectable levels (< 0.2 ng/mL) in 68% of patients with mCSPC1
- The PSA undetectable rate was 68.1% (95% CI, 63.9-72.1) in the XTANDI + GnRH therapy† group and 17.6%‡ (95% CI, 14.4-21.2) in the placebo + GnRH therapy† group6
- In patients with a detectable PSA level at baseline, treatment with XTANDI + GnRH therapy† significantly increased the chance of a PSA decline to an undetectable level (< 0.2 ng/mL) compared to treatment with placebo + GnRH therapy† by 50.5% (95% CI, 45.3-55.7; P < 0.0001)‡6
Key secondary endpoint from primary analysis: Time to deterioration of urinary symptoms§6
Treatment with XTANDI + GnRH therapy† was not associated with a statistically significant change in the time to deterioration of urinary symptoms compared with placebo + GnRH therapy† (HR = 0.88 [95% CI, 0.72-1.08]; P = 0.2162).‡
*PSA undetectable rate was defined as the percentage of patients with undetectable (< 0.2 ng/mL) PSA values at any time during study treatment of those patients with detectable (≥ 0.2 ng/mL) PSA values at baseline.6
†Or after bilateral orchiectomy.1
‡This information is not included in the US Full Prescribing Information for XTANDI.
§A deterioration in urinary symptoms was defined as an increase in the urinary symptoms subscale score by ≥ 50% of the standard deviation observed in the urinary symptoms subscale score at baseline.2
Time to castration resistance was also evaluated in patients with mCSPC5
The information below is not included in the US Full Prescribing Information for XTANDI. This secondary endpoint was prespecified but not alpha protected and should be considered descriptive information.
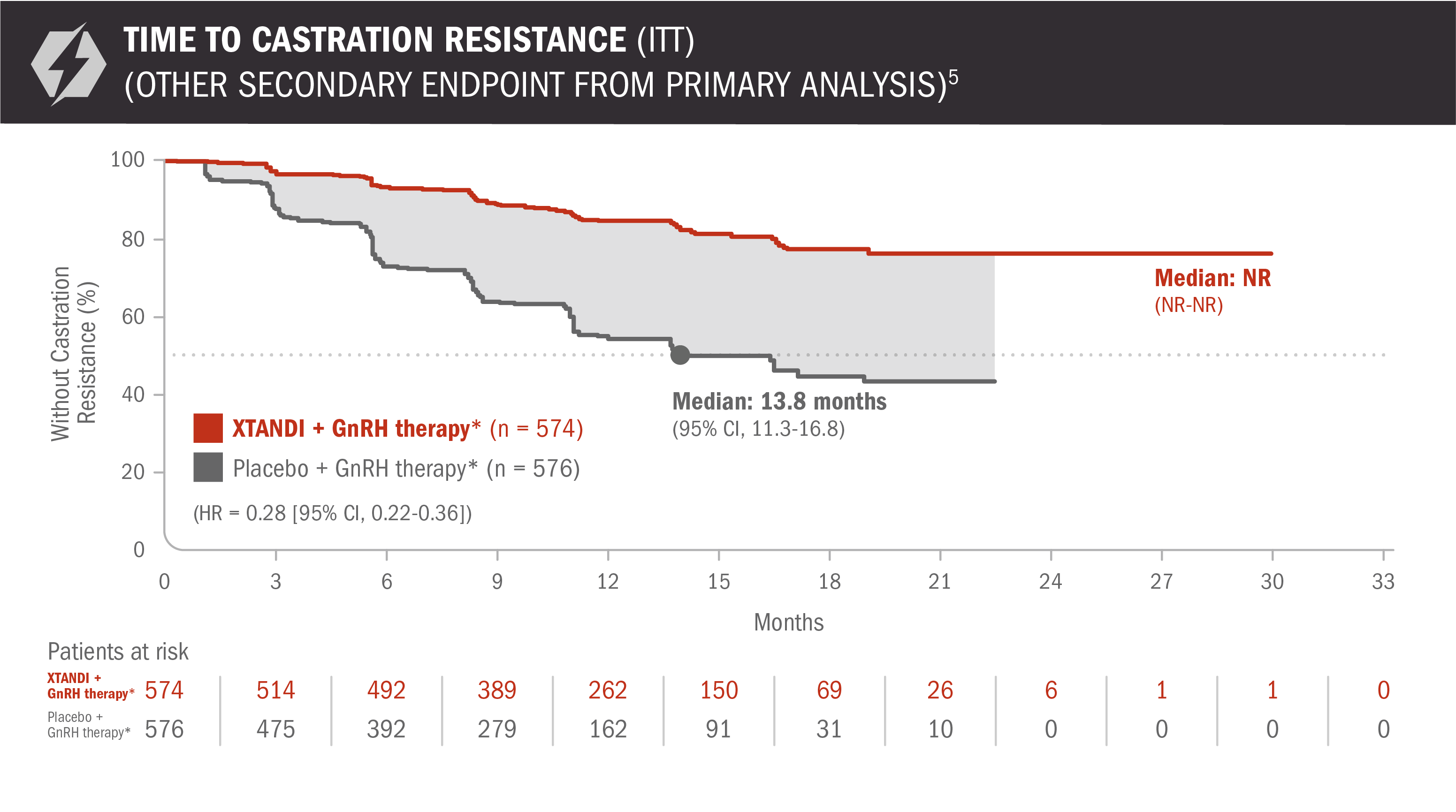
- Number of events: 90 (15.68%) with XTANDI + GnRH therapy* and 257 (44.62%) with placebo + GnRH therapy*6
Time to castration resistance was defined as the time from randomization to the first castration-resistant event (radiographic disease progression, PSA progression, or symptomatic skeletal event with castrate levels of testosterone [< 50 ng/dL]), whichever occurred first.5
*Or after bilateral orchiectomy.1
A post-hoc analysis of time to castration resistance was performed at the time of final overall survival analysis4,7
The information below is not included in the US Full Prescribing Information for XTANDI. An update to the analysis of time to castration resistance was performed at the time of the final overall survival analysis. There was no assessment of statistical significance for this post-hoc analysis, and it should be understood as descriptive information.
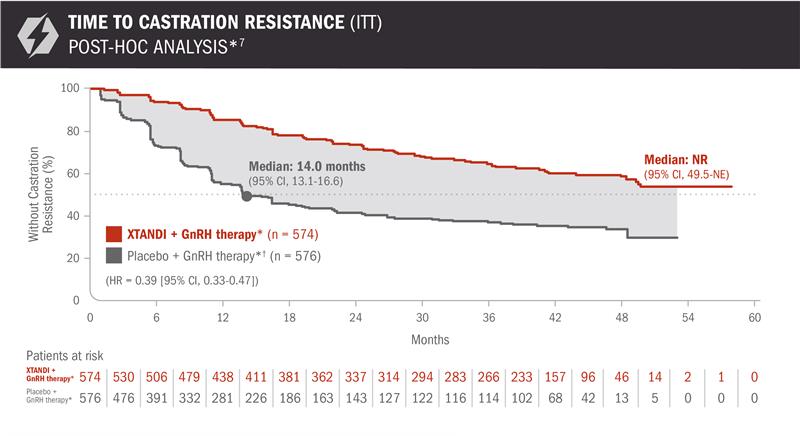
- Number of events: 202 (35.2%) with XTANDI + GnRH therapy* and 327 (56.8%) with placebo + GnRH therapy*6
*Or after bilateral orchiectomy.1
†In ARCHES, after unblinding, 184 patients (31.9%) randomly assigned to placebo + GnRH therapy* remained progression free and consented to crossover, 180 (31.3%) of whom received treatment with XTANDI + GnRH therapy*. The median time to crossover was 21.5 months.4
XTANDI in mCSPC
(ARCHES trial
publication)
XTANDI in mCSPC (ARCHES
trial publication)
Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/JCO.19.00799.
View the PublicationOverall survival: XTANDI + GnRH therapy* and NSAA† + GnRH therapy* in patients with mCSPC9
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. The overall patient population in ENZAMET included patients who received or did not receive concomitant docetaxel. The efficacy and safety of XTANDI in combination with docetaxel has not been established. ENZAMET was neither designed nor powered to analyze the results of overall survival in the individual subgroups. Therefore, an improvement in overall survival cannot be established in any subgroup, including mCSPC patients taking XTANDI + GnRH therapy* alone or with concomitant docetaxel. The updated analysis of the primary endpoint was prespecified but not alpha protected and should be considered descriptive information.
30% reduction in the risk of death with XTANDI + GnRH therapy* vs NSAA + GnRH therapy*9
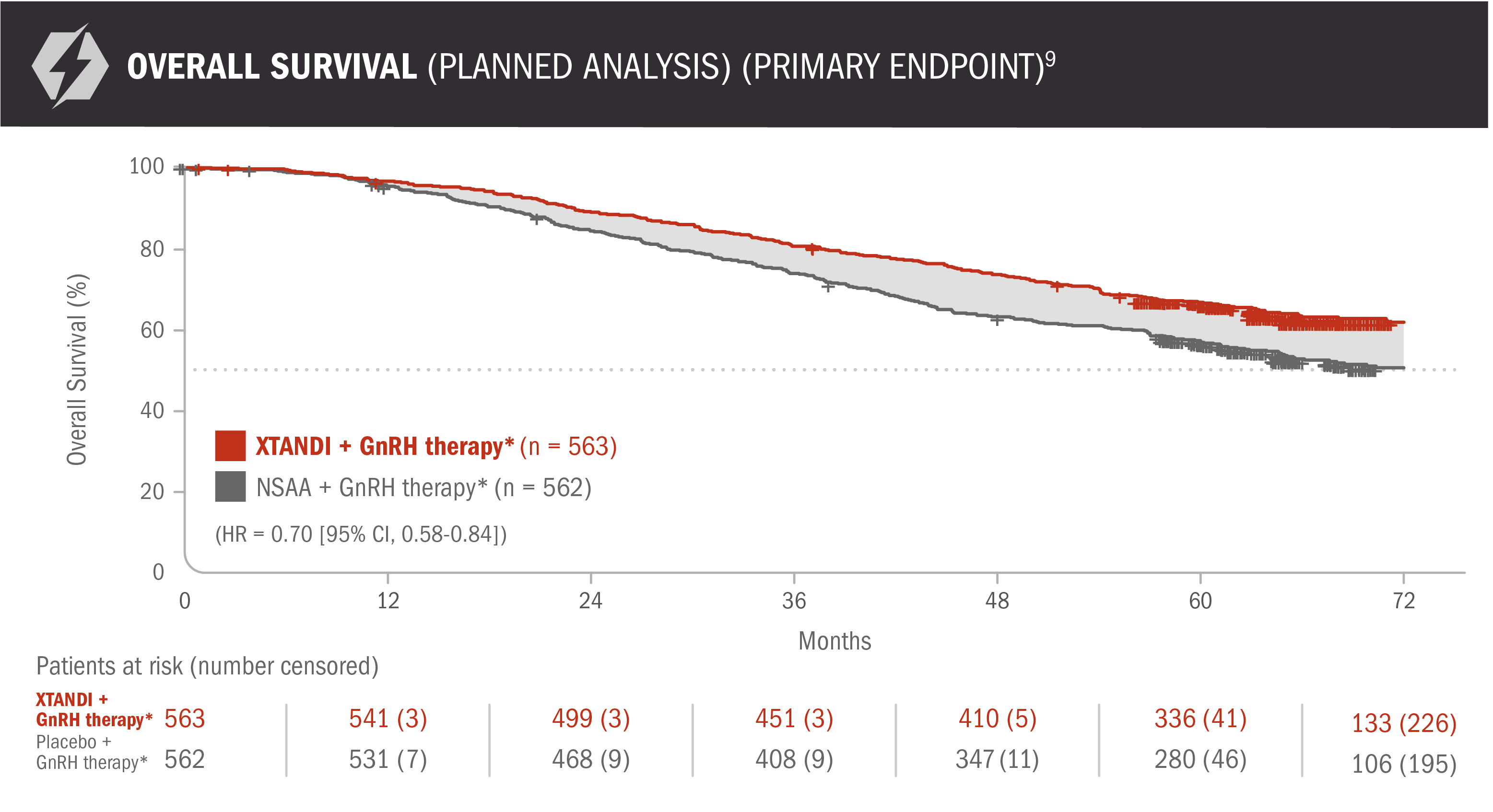
- Number of events: 208 (37%) with XTANDI + GnRH therapy* and 268 (48%) with NSAA + GnRH therapy*9
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
Concomitant docetaxel subgroup analyses
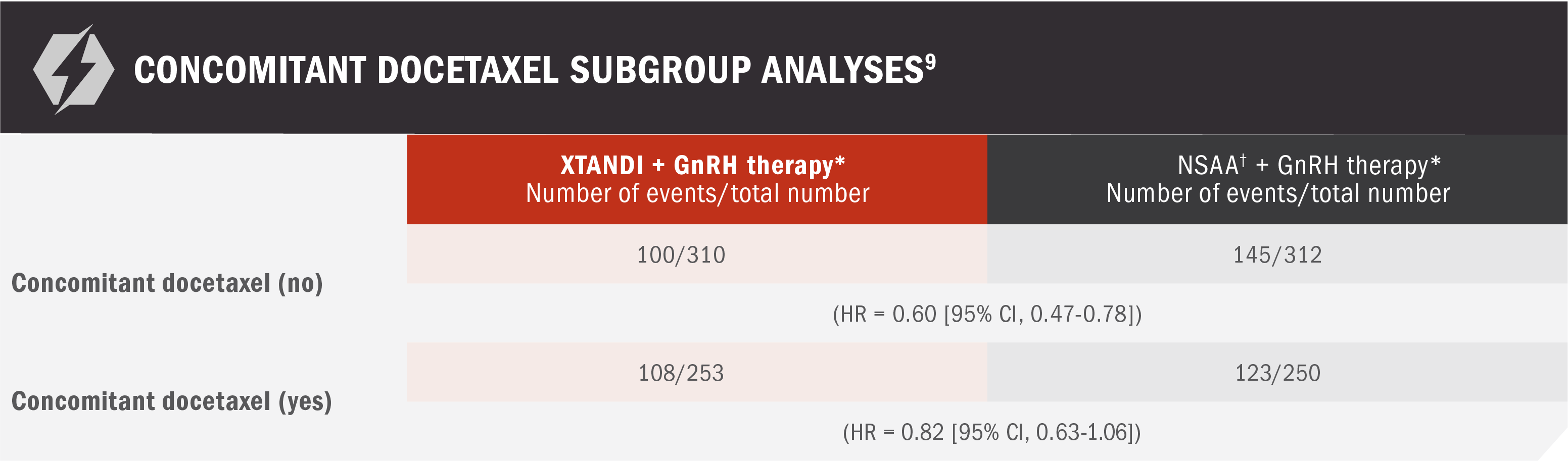
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
See mCSPC (ENZAMET) Adverse Reactions
XTANDI + GnRH therapy* in mCSPC was assessed in another large phase 3 study (ENZAMET)8
ENZAMET was an open-label study assessing XTANDI + GnRH therapy* and NSAA† + GnRH therapy* in patients with mCSPC8
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. The overall patient population in ENZAMET included those who received or did not receive concomitant docetaxel, and the efficacy and safety of XTANDI in combination with docetaxel is not established.
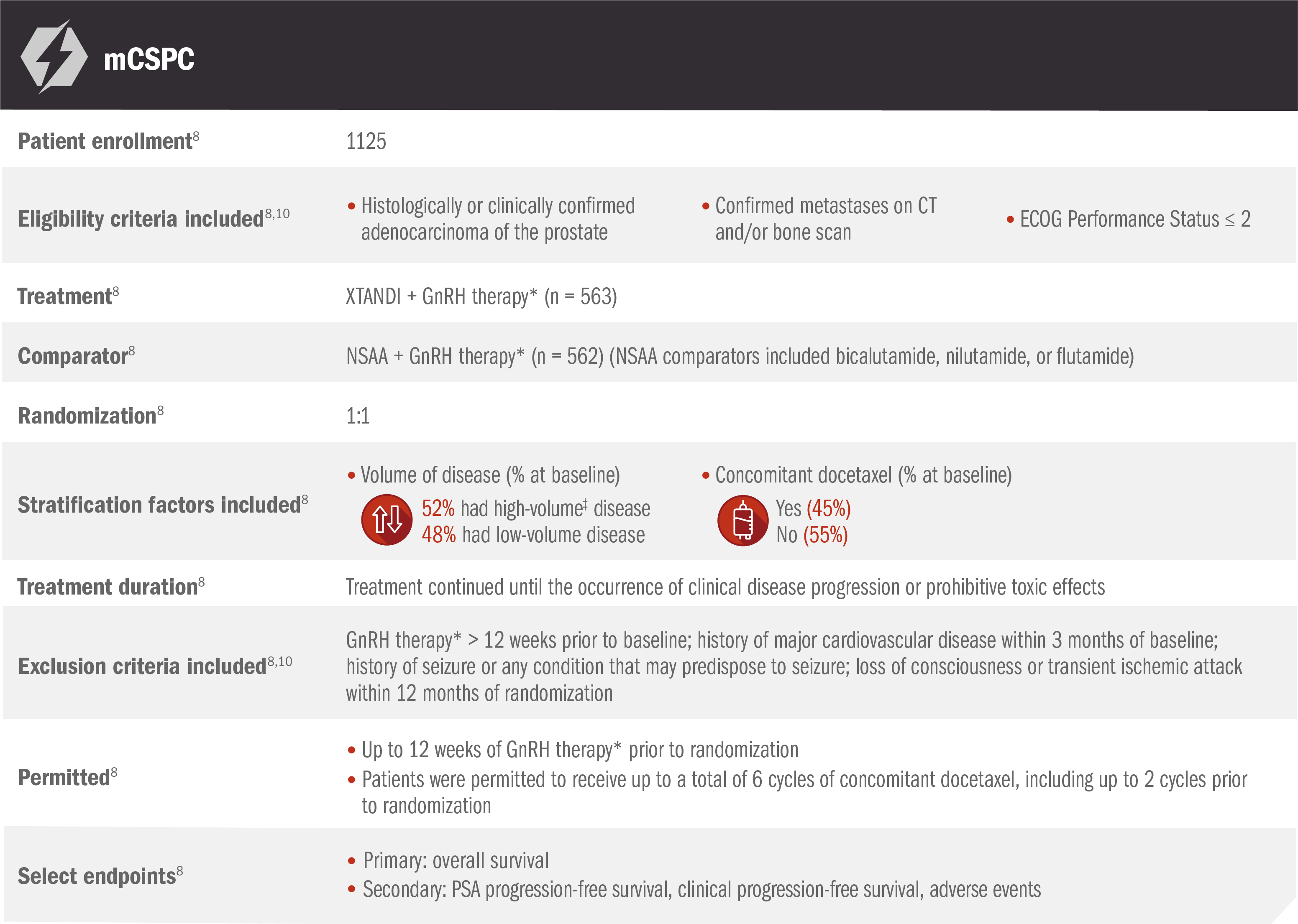
The primary endpoint of overall survival was measured as the interval from randomization to death from any cause or to the date of last known follow-up.9
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
‡Defined as metastases involving the viscera or, in the absence of visceral lesions, ≥ 4 bone lesions, ≥ 1 of which must be in a bony structure beyond the vertebral column and pelvic bone.8
Overall survival: prespecified subgroup analysis in patients with mCSPC9
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. Subgroups were prespecified but not alpha protected and should be considered exploratory analyses. These subgroup analyses are presented for descriptive purposes and cannot be interpreted as a demonstration of efficacy in any particular subgroup.
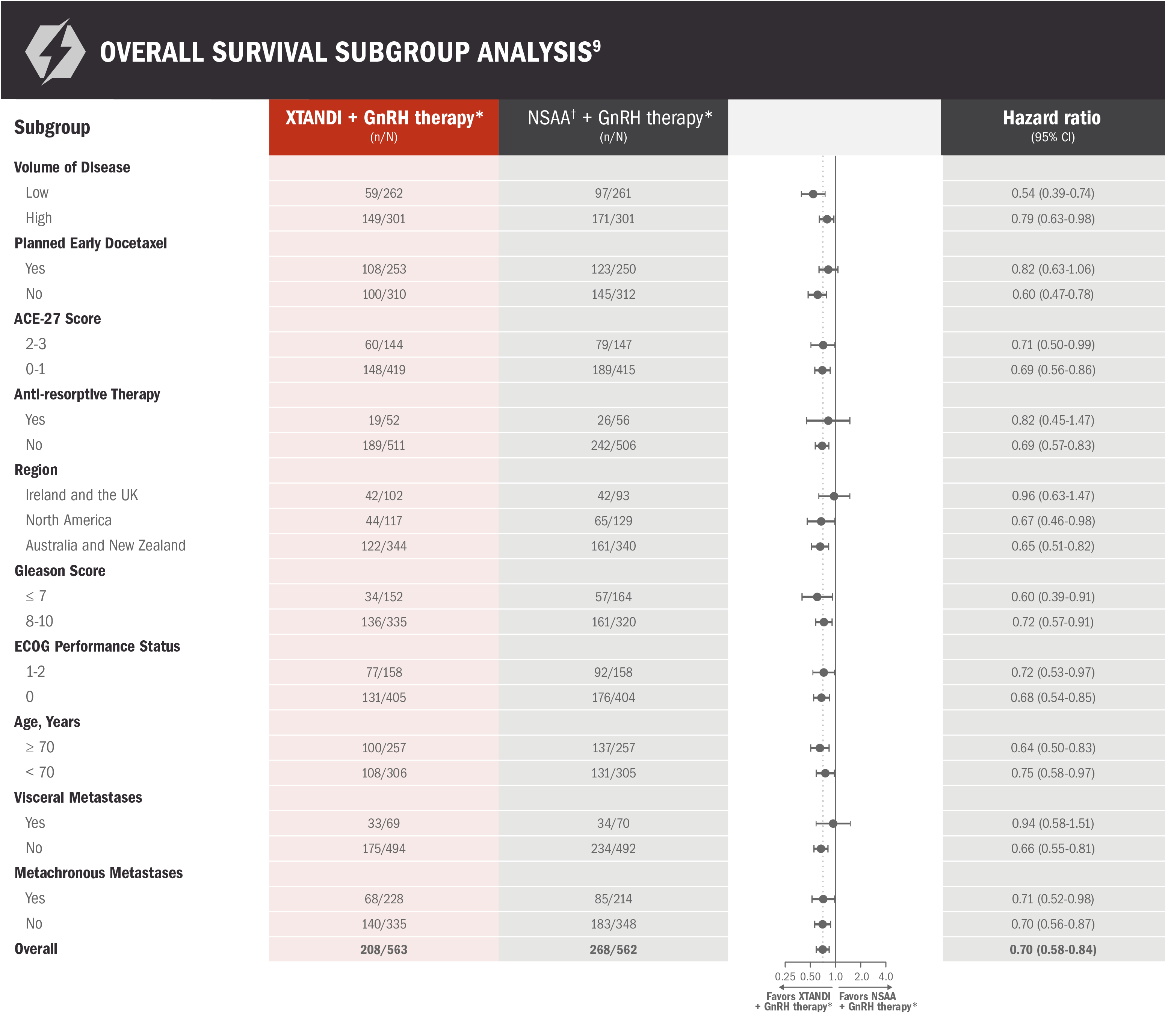
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
PSA progression-free survival: XTANDI + GnRH therapy* and NSAA† + GnRH therapy* in patients with mCSPC9
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. The overall patient population in ENZAMET included patients who received or did not receive concomitant docetaxel. The efficacy and safety of XTANDI in combination with docetaxel has not been established.
PSA is not a reliable surrogate for overall survival. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.
An update to the analysis of time to PSA progression was performed at the time of the updated overall survival analysis. There was no assessment of statistical significance, and it should be understood as descriptive information.
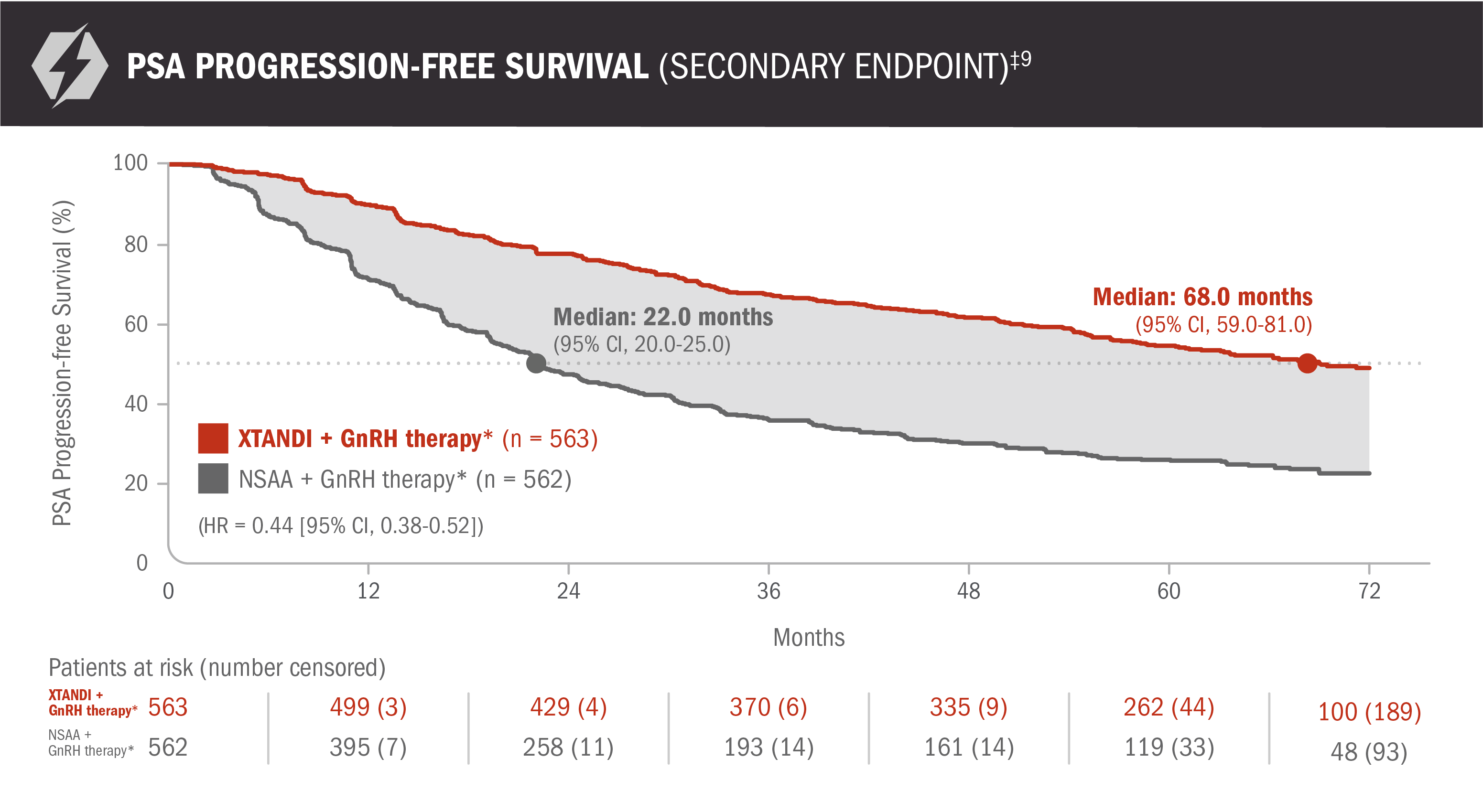
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
‡PSA progression-free survival was defined as time from randomization to the earliest PSA progression according to the PCWG2 criteria, clinical progression, or death from any cause, whichever occurred first, or the last known date of follow-up without PSA progression.9
Clinical progression-free survival: XTANDI + GnRH therapy* and NSAA† + GnRH therapy* in patients with mCSPC9
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. The overall patient population in ENZAMET included patients who received or did not receive concomitant docetaxel. The efficacy and safety of XTANDI in combination with docetaxel has not been established.
An update to the analysis of clinical progression was performed at the time of the updated overall survival analysis. There was no assessment of statistical significance, and it should be understood as descriptive information.
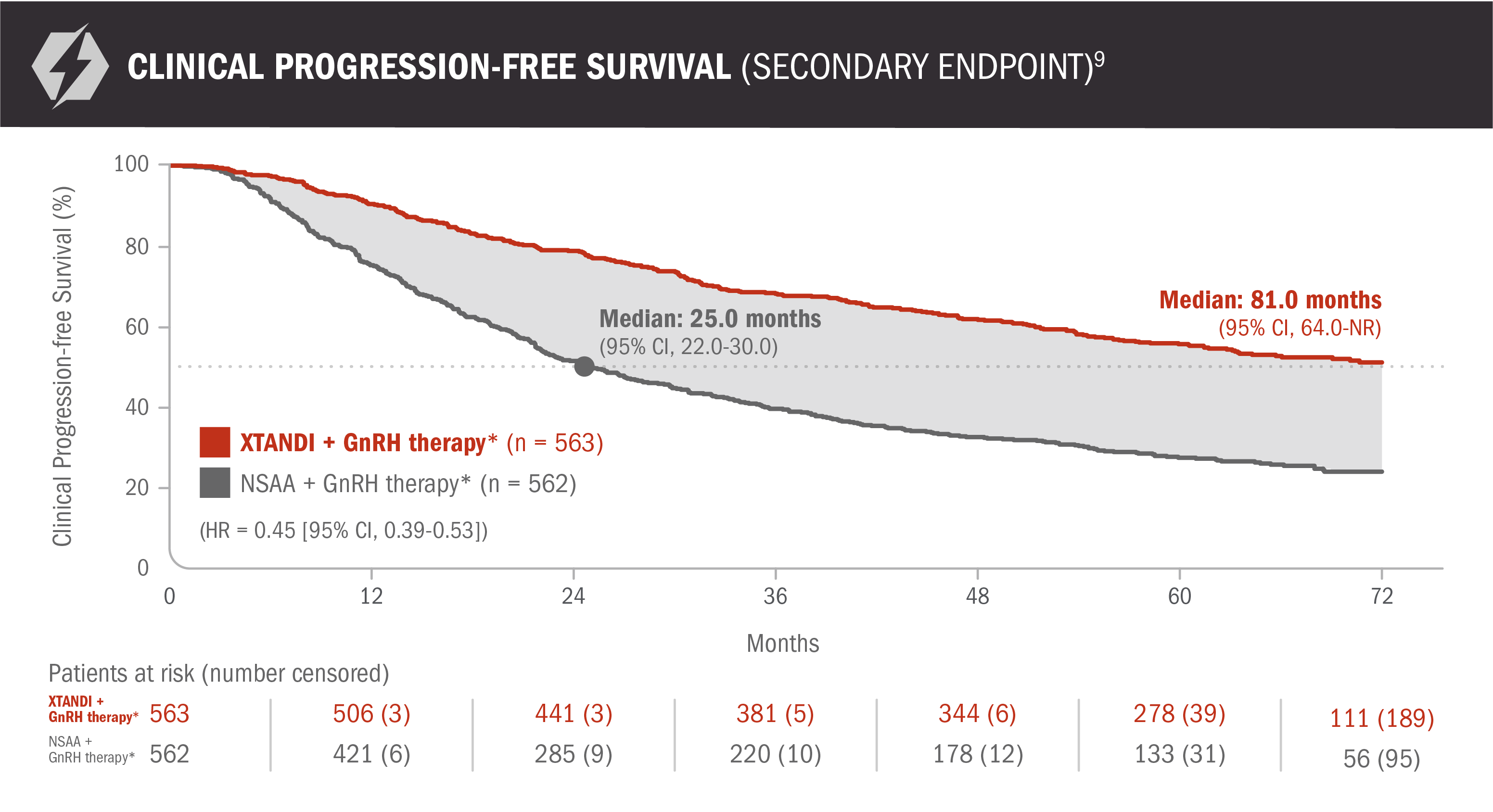
Clinical progression-free survival was defined as the earliest sign of radiographic progression using PCWG2 for bone lesions and RECIST 1.1 for soft-tissue lesions, symptoms attributable to cancer progression, or initiation of another anticancer treatment for prostate cancer.9
*Or after bilateral orchiectomy.10
†Conventional NSAAs: bicalutamide 50 mg/day, nilutamide 150 mg/day, or flutamide 250 mg 3 times a day.10
about XTANDI in mCSPC (ENZAMET trial publication)
XTANDI in mCSPC (ENZAMET
trial publication)
Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835.
View The PublicationEMBARK Trial
XTANDI + GnRH therapy* significantly improved metastasis-free survival vs placebo + GnRH therapy*1
EMBARK was a randomized phase 3 trial that assessed the efficacy and safety of XTANDI + GnRH therapy* vs placebo + GnRH therapy* in 1068 patients with nmCSPC with high-risk BCR.1,2
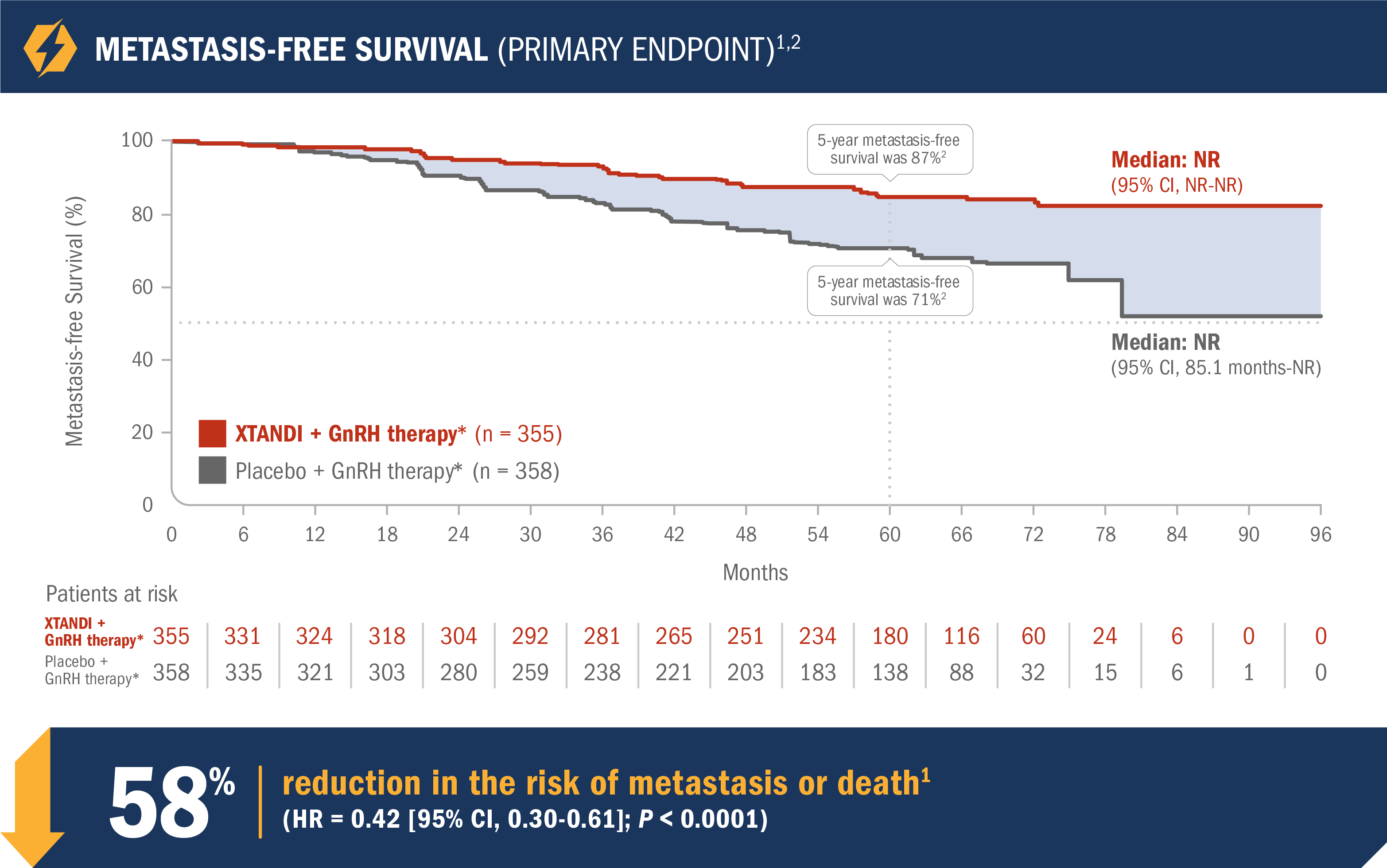
- Number of events: 45 (12.7%) with XTANDI + GnRH therapy* vs 92 (25.7%) with placebo + GnRH therapy*1
- Median metastasis-free survival was not reached in either the XTANDI + GnRH therapy* arm or the placebo + GnRH therapy* arm1
- The 5-year metastasis-free survival was 87% in the XTANDI + GnRH therapy* arm and 71% in the placebo + GnRH therapy* arm. This time point was not prespecified and is not in the US Full Prescribing Information for XTANDI1,2
See nmCSPC with high-risk BCR (EMBARK) adverse reactions
*Leuprolide.1
The EMBARK trial assessed the efficacy and safety of XTANDI with or without GnRH therapy* vs placebo + GnRH therapy* in patients with nmCSPC with high-risk BCR1

In the EMBARK trial, patients were required to have nonmetastatic disease by BICR, high-risk BCR (defined by a PSA doubling time ≤ 9 months), and PSA values ≥ 1 ng/mL if they had prior RP (with or without RT) as the primary treatment for prostate cancer or PSA values ≥ 2 ng/mL above the nadir if they had prior RT only.1
*Leuprolide.1
†All treatment arms were eligible for treatment suspension. In the XTANDI + GnRH therapy* and placebo + GnRH therapy* arms, GnRH therapy* was also suspended.1
‡Study treatment was suspended once at Week 37 if PSA was < 0.2 ng/mL at Week 36; treatment was reinitiated when PSA values increased to ≥ 2.0 ng/mL for patients with prior prostatectomy or ≥ 5.0 ng/mL for patients without prior prostatectomy.1
Patient demographics and baseline characteristics were balanced between the 3 treatment arms2
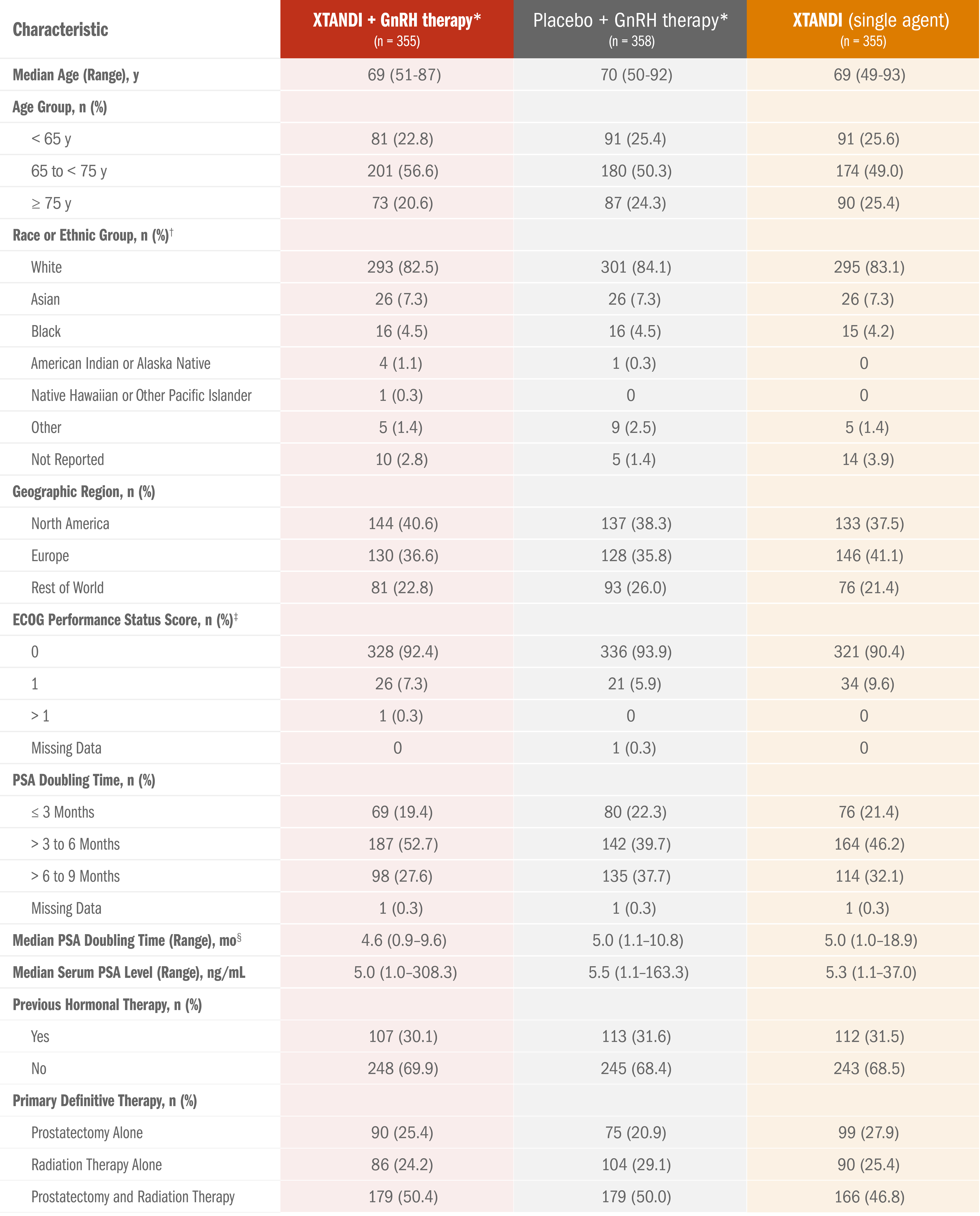
Percentages may not total 100 because of rounding.
*Leuprolide.1
†Race or ethnic group was reported by the patients. The "Other" category includes patients who identified as multiple races or ethnic groups.2
‡ECOG Performance Status scores range from 0 to 5, with higher scores indicating greater disability.2
§PSA doubling time at baseline was calculated on the basis of a sequence of PSA values tested over time during the enrollment period. Some baseline PSA doubling time values exceeded the enrollment threshold of less than 9 months owing to discrepancies in the PSA values captured in the case-report forms as compared with the values used for the enrollment calculation.2
XTANDI (single agent) significantly improved metastasis-free survival vs placebo + GnRH therapy*1
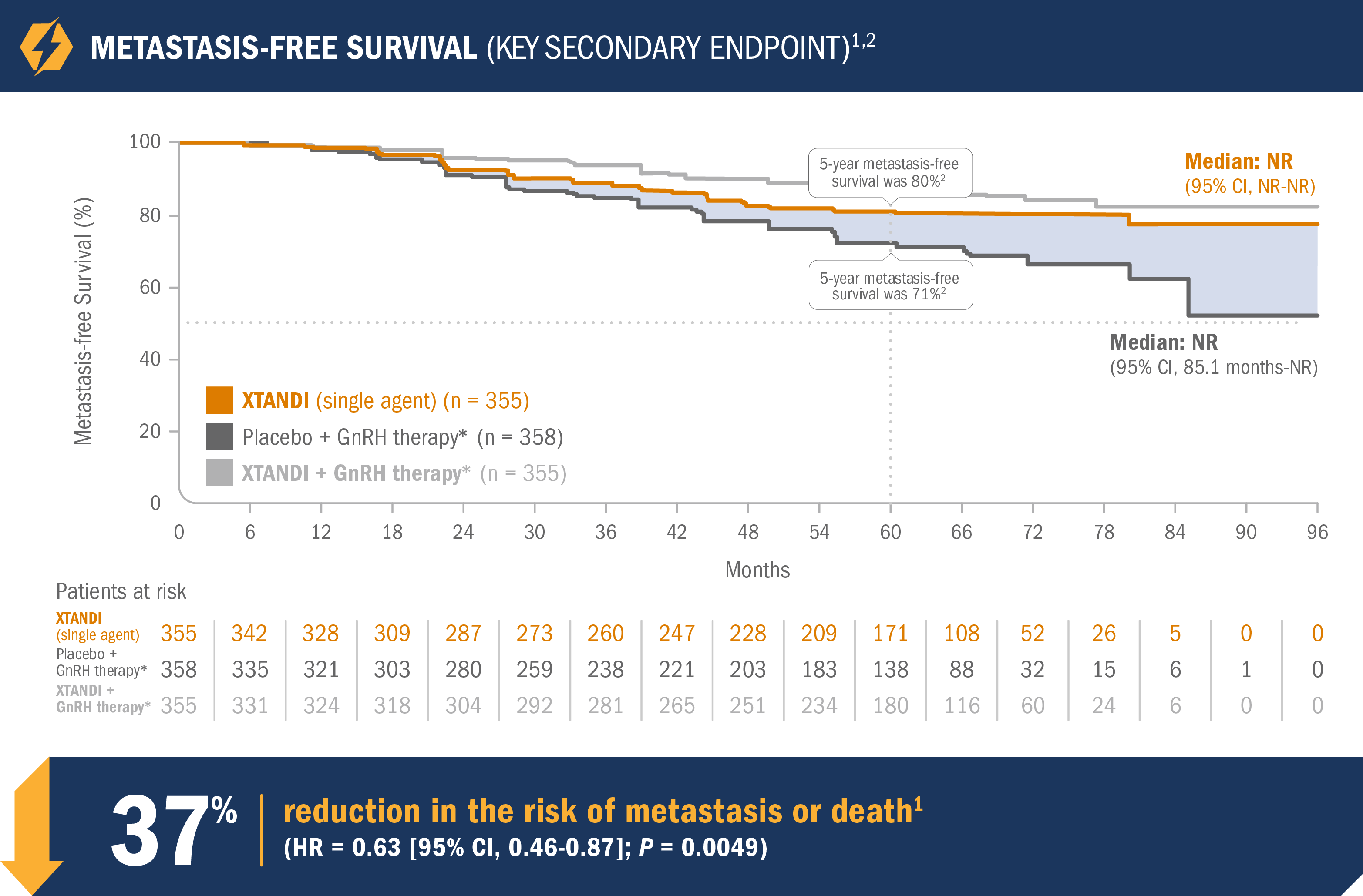
- Number of events: 63 (17.7%) with XTANDI (single agent) vs 92 (25.7%) with placebo + GnRH therapy*1
- Median metastasis-free survival was not reached in either the XTANDI (single agent) arm or the placebo + GnRH therapy* arm1
- The 5-year metastasis-free survival was 80% in the XTANDI (single agent) arm and 71% in the placebo + GnRH therapy* arm. This endpoint was not prespecified and is not in the US Full Prescribing Information for XTANDI1,2
*Leuprolide.1
Final analysis of overall survival in patients with nmCSPC with high-risk BCR4
This endpoint was a prespecified, alpha-protected, key secondary endpoint. This information is not included in the US Full Prescribing Information for XTANDI.
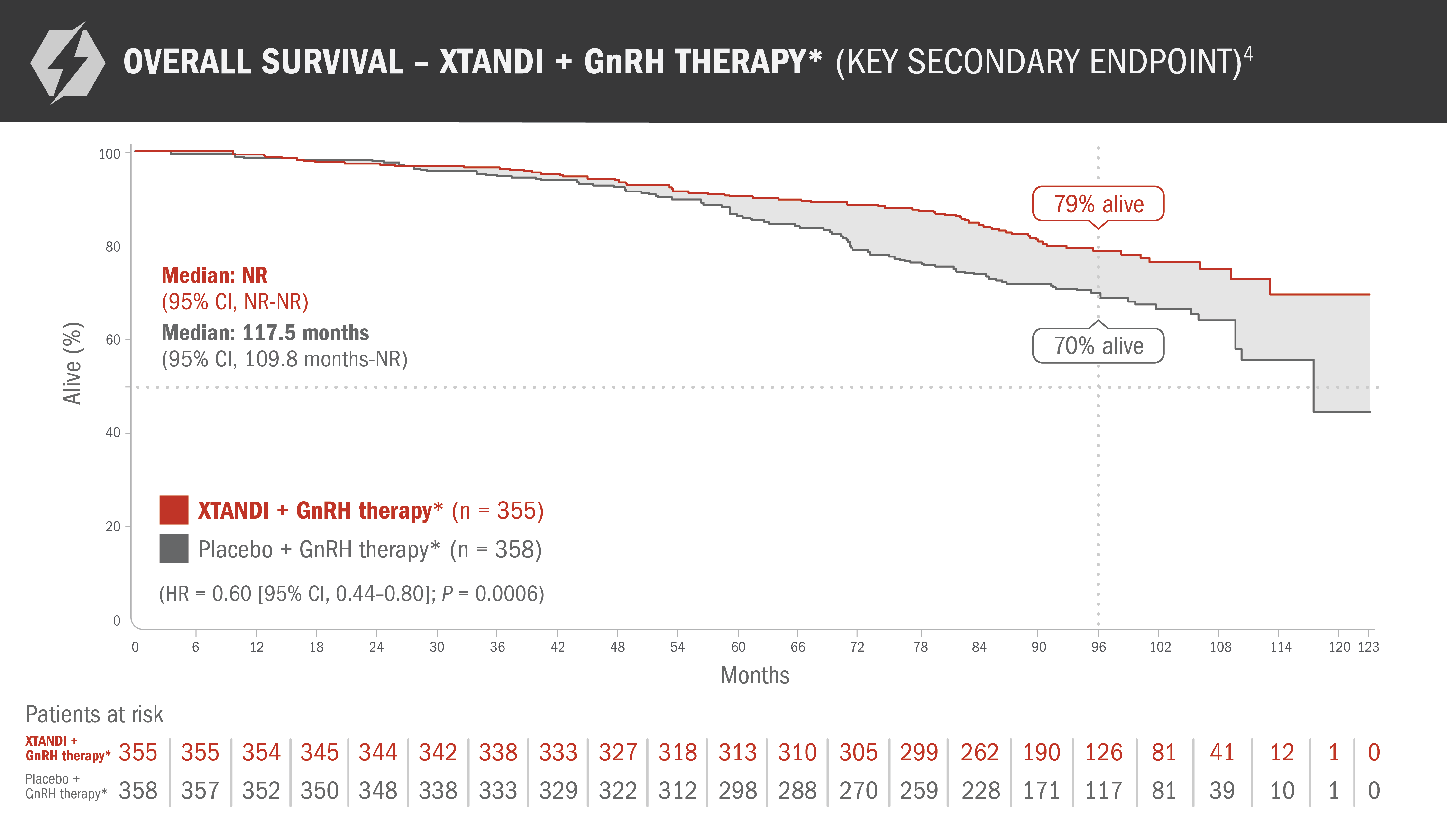
- Number of events: 73 (20.6%) with XTANDI + GnRH therapy* and 111 (31.0%) with placebo + GnRH therapy*4
- The median follow-up time was 94.2 months with XTANDI + GnRH therapy* and 94.0 months with placebo + GnRH therapy*4
- At 8 years, 79% of patients randomized to XTANDI + GnRH therapy* and 70% of patients randomized to placebo + GnRH therapy* were alive according to Kaplan-Meier calculations4
OVERALL SURVIVAL – XTANDI (SINGLE AGENT)4
The overall survival analysis between patients treated with XTANDI (single agent) and placebo + GnRH therapy* was not statistically significant (HR = 0.83 [95% CI, 0.63-1.10]; P = 0.19).4
*Leuprolide.1
Subgroups were prespecified but not alpha protected and should be considered exploratory analyses. These subgroup analyses are presented for descriptive purposes and cannot be interpreted as a demonstration of efficacy in any particular subgroup. Certain subgroups were not included in these analyses due to small numbers of events. The information below is not included in the US Full Prescribing Information for XTANDI.
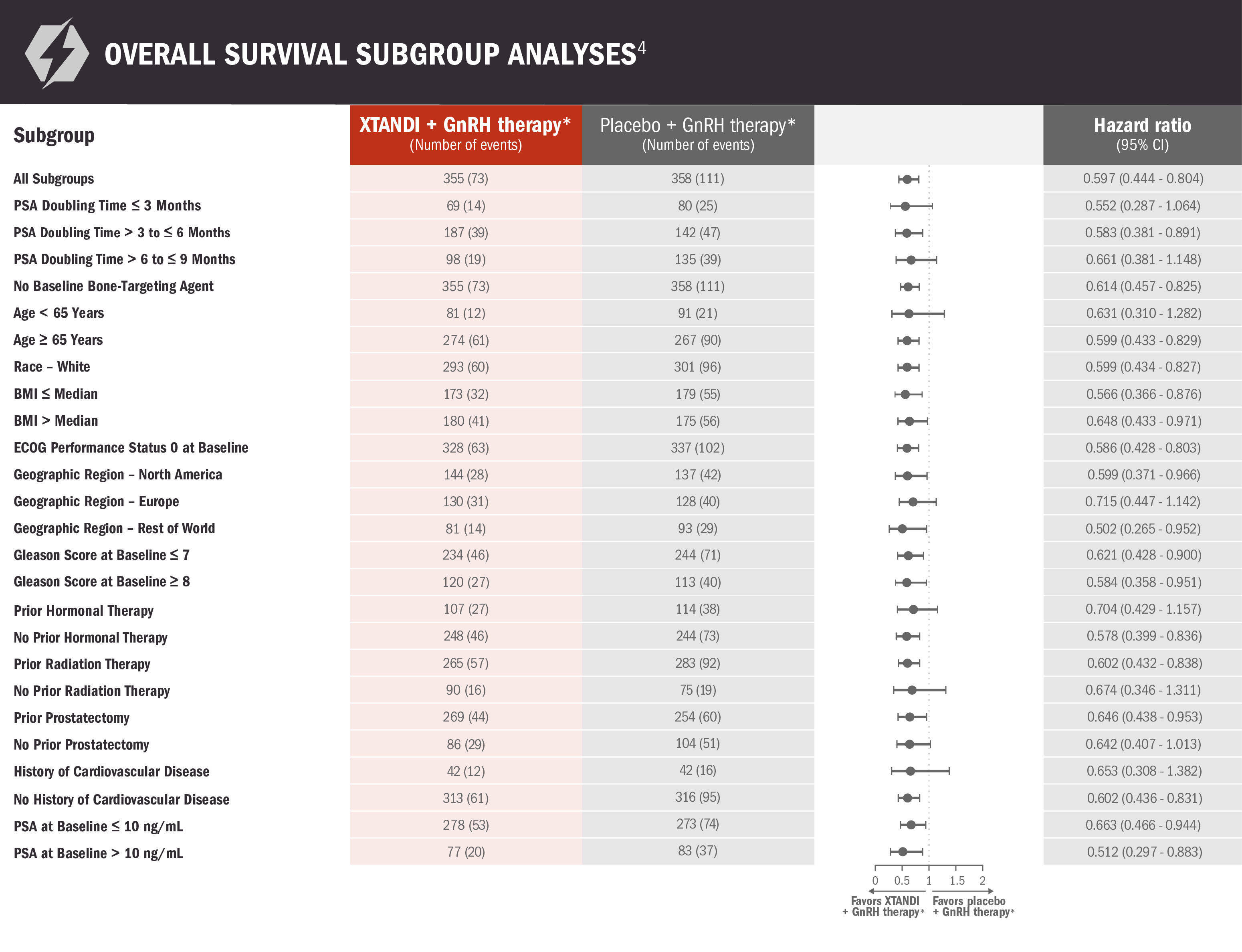
*Leuprolide.1
Time to start of new antineoplastic therapy was also assessed with XTANDI + GnRH therapy* and placebo + GnRH therapy*2,5
This endpoint was a prespecified, alpha-protected, key secondary endpoint. There is overlap of events between time to start of new antineoplastic therapy and metastasis-free survival, because patients with new metastases are generally started on a new antineoplastic drug. Hormonal therapy was the most frequent subsequent therapy received and accounted for approximately a quarter of patients who received any subsequent therapy. Less frequent subsequent therapies included cytotoxic therapies, prostate cancer vaccines, or systemic radiopharmaceuticals. This information should be considered when evaluating the clinical importance of this analysis. This information is not included in the US Full Prescribing Information for XTANDI.
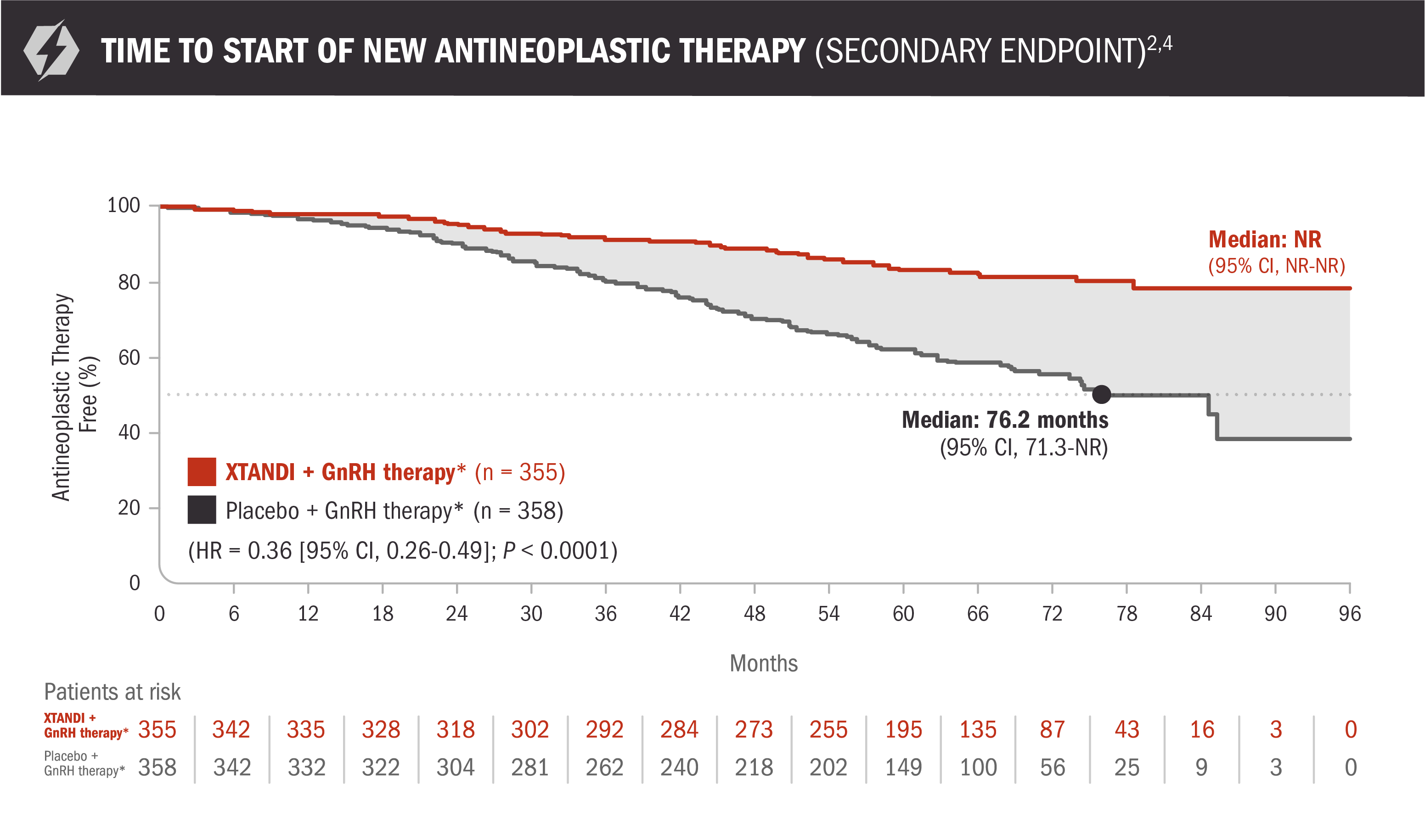
- Number of events: 58 (16%) with XTANDI + GnRH therapy* and 140 (39%) with placebo + GnRH therapy*2
- Median time to start of new antineoplastic therapy was not reached in the XTANDI + GnRH therapy* arm and was 76.2 months in the placebo + GnRH therapy* arm5
Time to start of new antineoplastic therapy was defined as the time from randomization to first use of a new antineoplastic therapy for prostate cancer. Subsequent prostate cancer therapies included hormonal therapies, cytotoxic therapies, prostate cancer vaccines, or systemic radiopharmaceuticals for prostate cancer.6
*Leuprolide.1
Treatment* was suspended if PSA dropped to undetectable at 36 weeks1
For patients whose PSA was undetectable (< 0.2 ng/mL) at Week 36, treatment* was suspended at Week 37 and then reinitiated when PSA increased to ≥ 2.0 ng/mL for patients with prior prostatectomy or ≥ 5.0 ng/mL for patients without prior prostatectomy.1
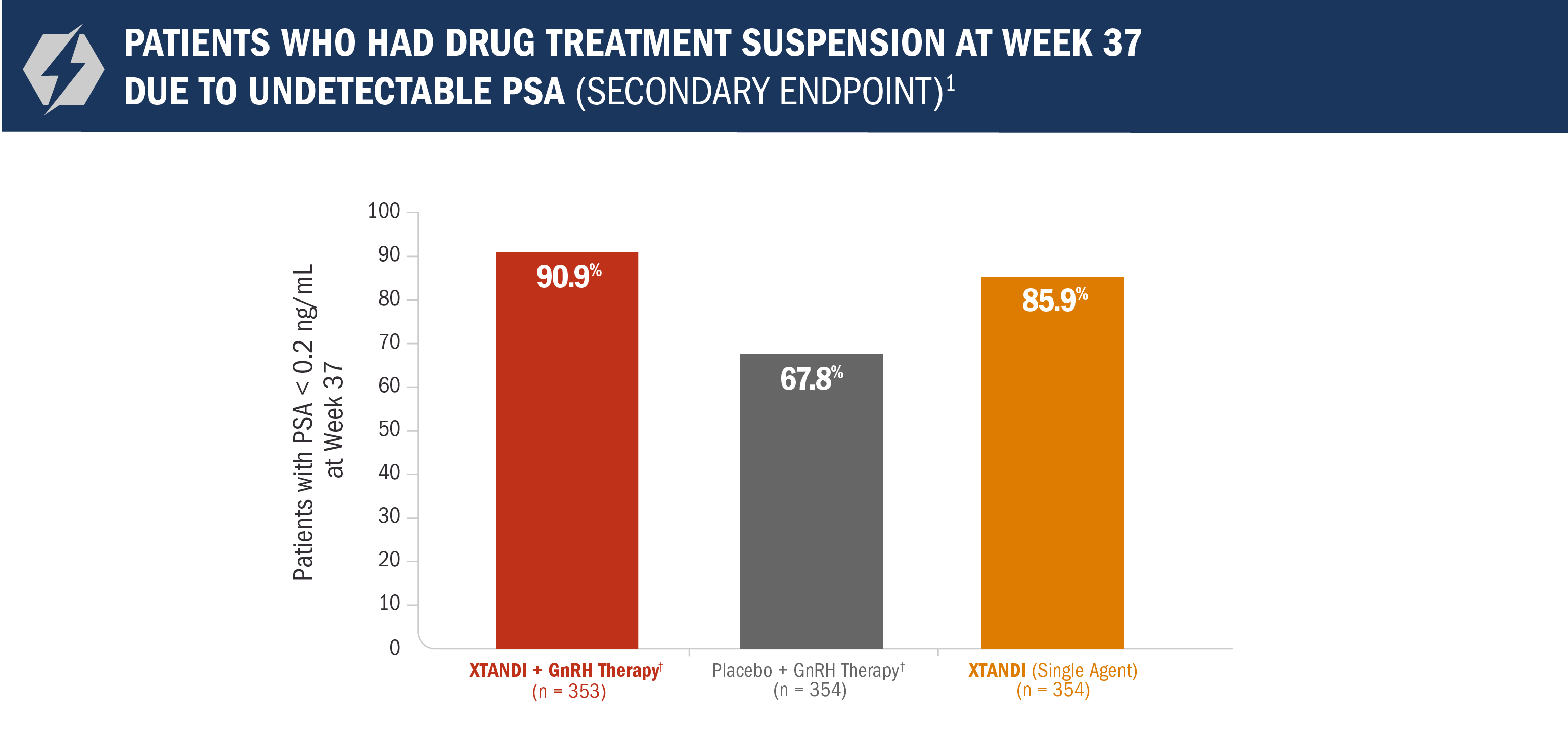
- Approximately 91% of patients in the XTANDI + GnRH therapy† arm reached undetectable PSA levels (< 0.2 ng/mL) at 9 months1
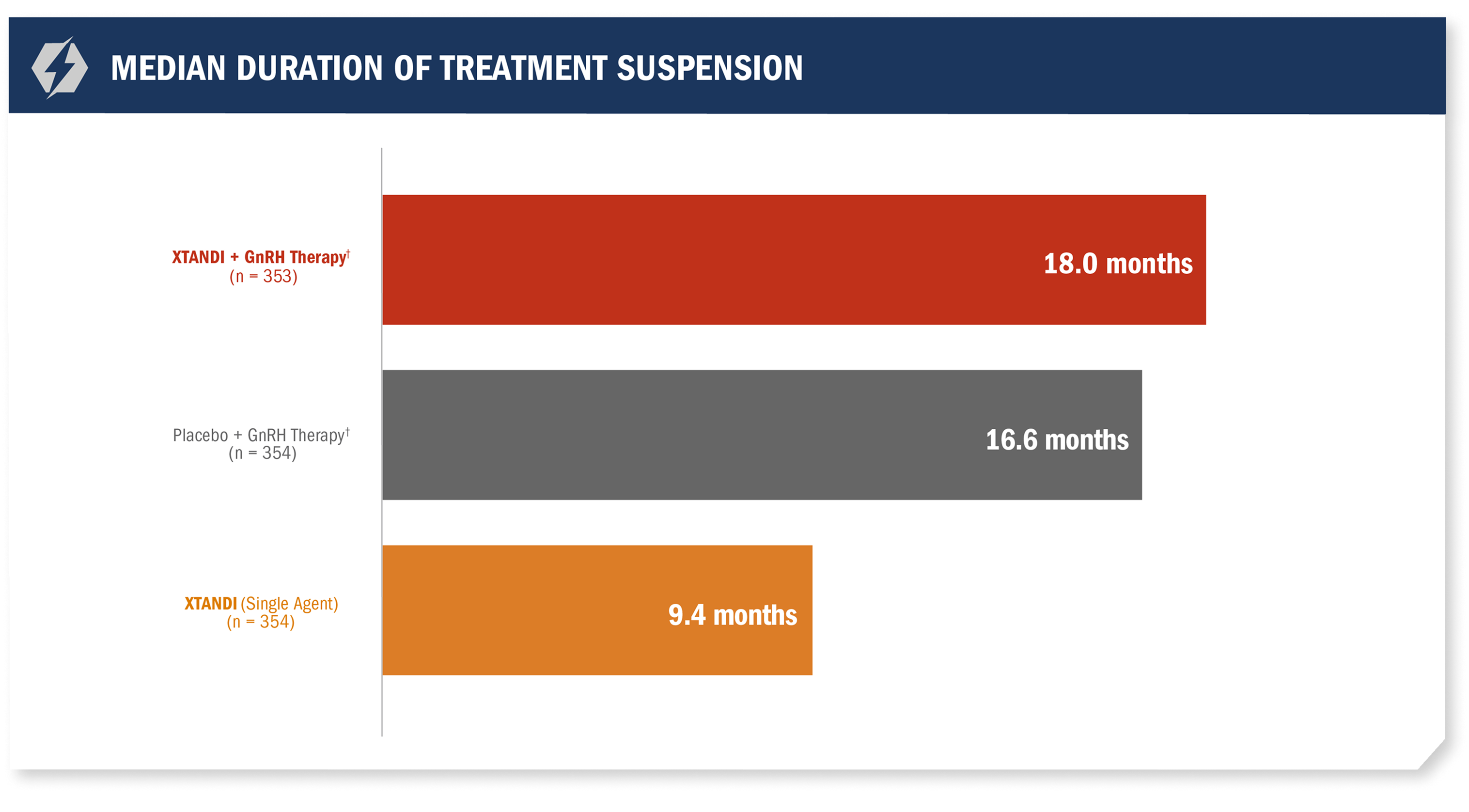
- Median treatment suspension duration in the XTANDI + GnRH therapy† arm due to undetectable PSA (< 0.2 ng/mL) at 36 weeks was 18 months1
*All treatment arms were eligible for treatment suspension. In the XTANDI + GnRH therapy† and placebo + GnRH therapy† arms, GnRH therapy† was also suspended.1
†Leuprolide.1
XTANDI in nmCSPC with
high-risk BCR (EMBARK
trial publication)
Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389(16):1453-1465. doi:10.1056/NEJMoa2303974.
View The PublicationBCR, biochemical recurrence; BICR, blinded independent central review; CI, confidence interval; CSPC, castration-sensitive prostate cancer; ECOG, Eastern Cooperative Oncology Group; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; mCSPC, metastatic castration-sensitive prostate cancer; MFS, metastasis-free survival; nmCSPC, nonmetastatic castration-sensitive prostate cancer; NR, not reached; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
References: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025. 2. Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389(16):1453-1465. doi:10.1056/NEJMoa2303974. 3. Freedland SJ, De Giorgi U, Gleave M, et al. A phase 3 randomised study of enzalutamide plus leuprolide and enzalutamide monotherapy in high-risk non-metastatic hormone-sensitive prostate cancer with rising PSA after local therapy: EMBARK study design [published online August 12, 2021]. BMJ Open. 2021. Accessed February 5, 2025. https://bmjopen.bmj.com/content/bmjopen/11/8/e046588.full.pdf. 4. Shore ND, de Almeida Luz M, De Giorgi U, et al. Improved survival with enzalutamide in biochemically recurrent prostate cancer [published online ahead of print October 19, 2025]. N Engl J Med. 2025. Accessed October 20, 2025. https://www.nejm.org/doi/full/10.1056/NEJMoa2510310. 5. Astellas. XTANDI. Data on File. 6. Supplement to: Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389(16):1453-1465. doi:10.1056/NEJMoa2303974.
ACE, adult comorbidity evaluation; CI, confidence interval; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FDA, U.S. Food and Drug Administration; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; ITT, intent to treat; mCSPC, metastatic castration-sensitive prostate cancer; NE, not evaluable; nmCSPC, nonmetastatic castration-sensitive prostate cancer; NR, not reached; NSAA, nonsteroidal anti-androgen; PCWG2, Prostate Cancer Working Group 2; PSA, prostate-specific antigen; RANKL, receptor activator of nuclear factor; RECIST 1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
References: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025. 2. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/JCO.19.00799. 3. Protocol for: Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/JCO.19.00799. 4. Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622. doi:10.1200/JCO.22.00193. 5. Supplement to: Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/JCO.19.00799. 6. Astellas. XTANDI. Data on File. 7. Supplement to: Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622. doi:10.1200/JCO.22.00193. 8. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835. 9. Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334. doi:10.1016/S1470-2045(23)00063-3. 10. Protocol for: Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835.