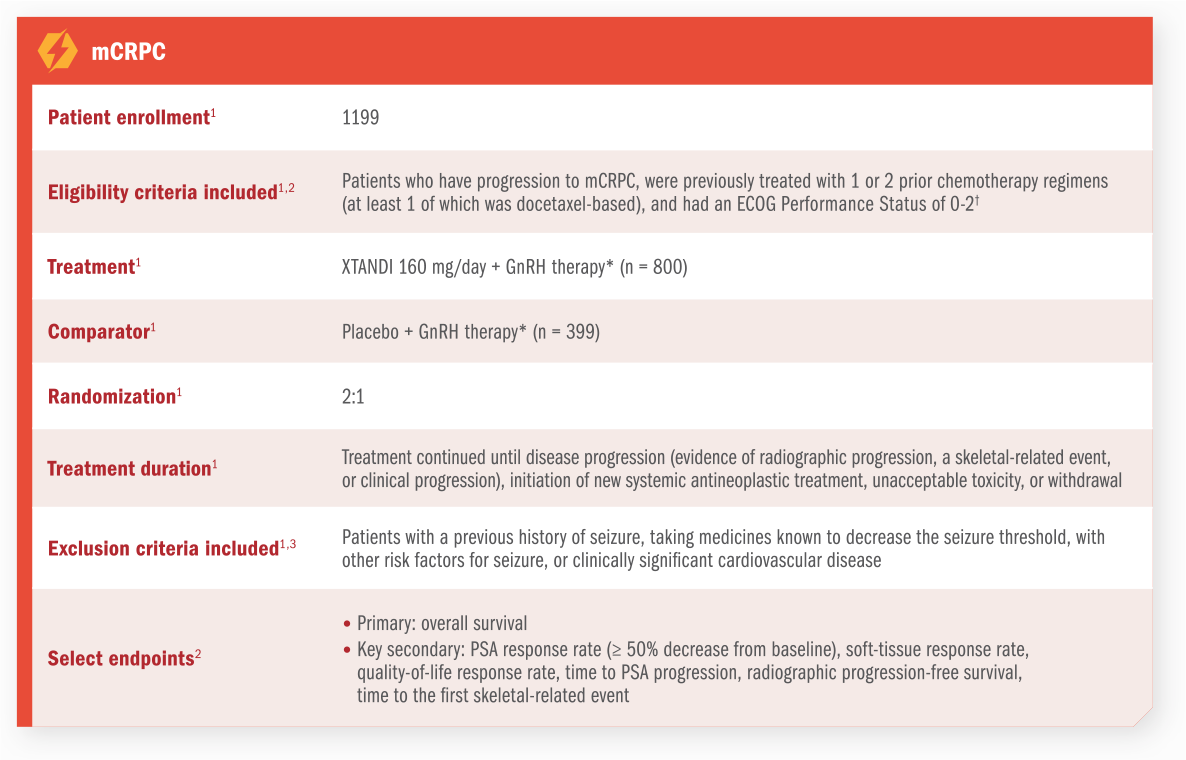
XTANDI has an established safety profile in mCSPC (ARCHES Trial)1
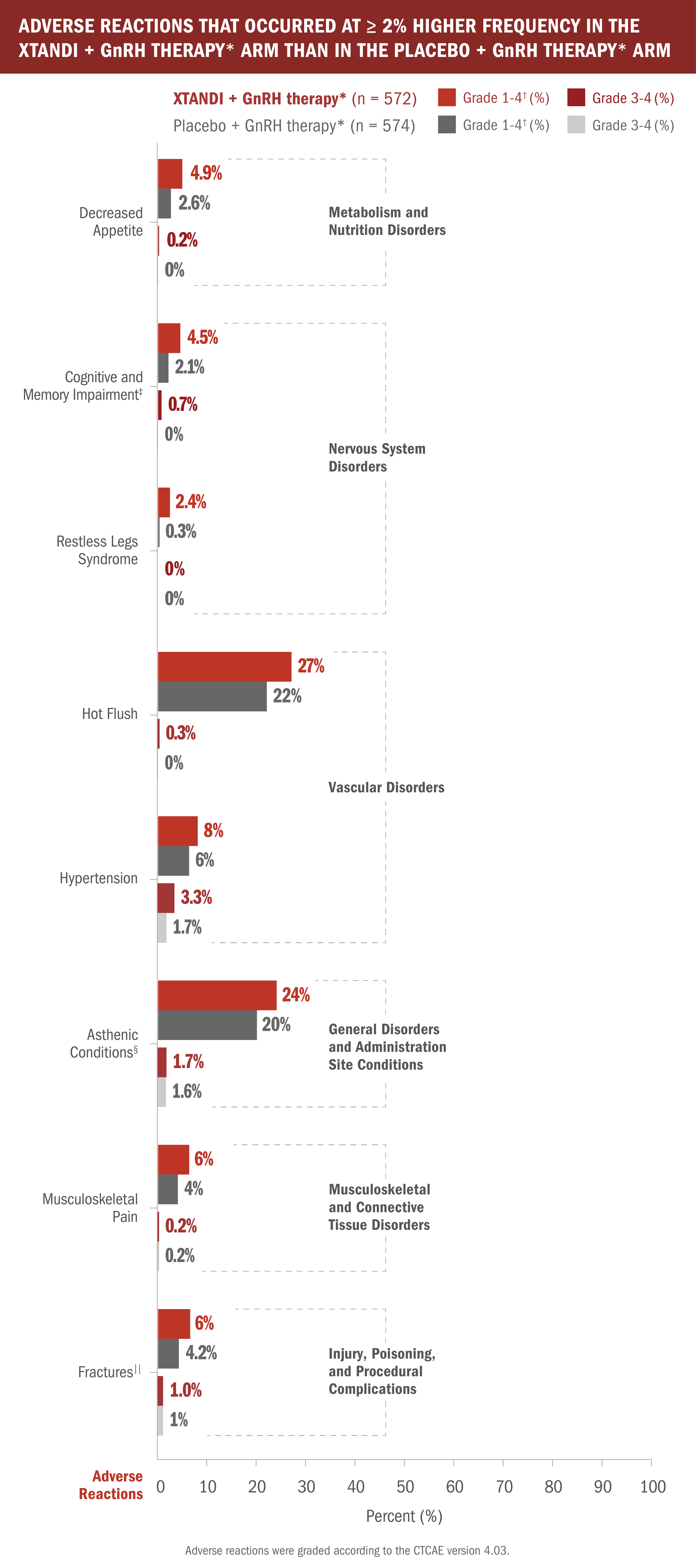
- The percentage of XTANDI + GnRH therapy*–treated patients who reported Grade 1-4 asthenic conditions was 24% vs 20% with placebo + GnRH therapy.* The percentage of XTANDI + GnRH therapy*–treated patients who reported Grade 3-4 asthenic conditions was 1.7% vs 1.6% with placebo + GnRH therapy*
- The median duration of treatment was 12.8 months (range: 0.2-26.6 months) with XTANDI + GnRH therapy* and 11.6 months (range: 0.2-24.6 months) with placebo + GnRH therapy*
*Or after bilateral orchiectomy.1
†CTCAE version 4.03.
‡Includes memory impairment, amnesia, cognitive disorder, dementia, disturbance in attention, transient global amnesia, dementia Alzheimer's type, mental impairment, senile dementia, and vascular dementia.
§Includes asthenia and fatigue.
‖Includes fracture-related preferred terms under high-level terms, fractures NEC, fractures and dislocations NEC, limb fractures and dislocations, pelvic fractures and dislocations, skull and brain therapeutic procedures, skull fractures, facial bone fractures and dislocations, spinal fractures and dislocations, and thoracic cage fractures and dislocations.
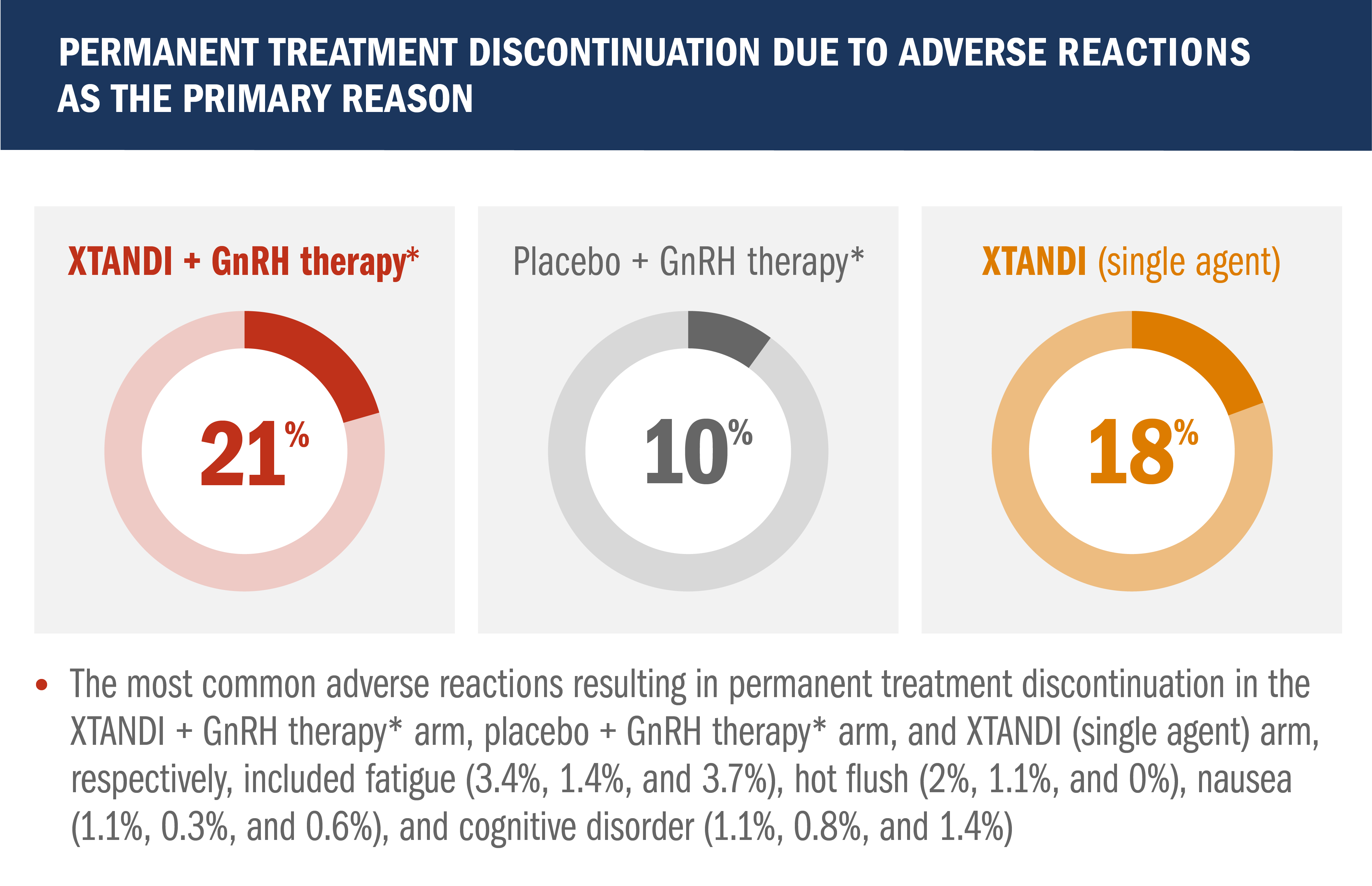
Permanent discontinuation due to adverse reactions as the primary reason was reported in 4.9% of XTANDI + GnRH therapy*–treated patients and 3.7% of placebo + GnRH therapy*–treated patients in this mCSPC study
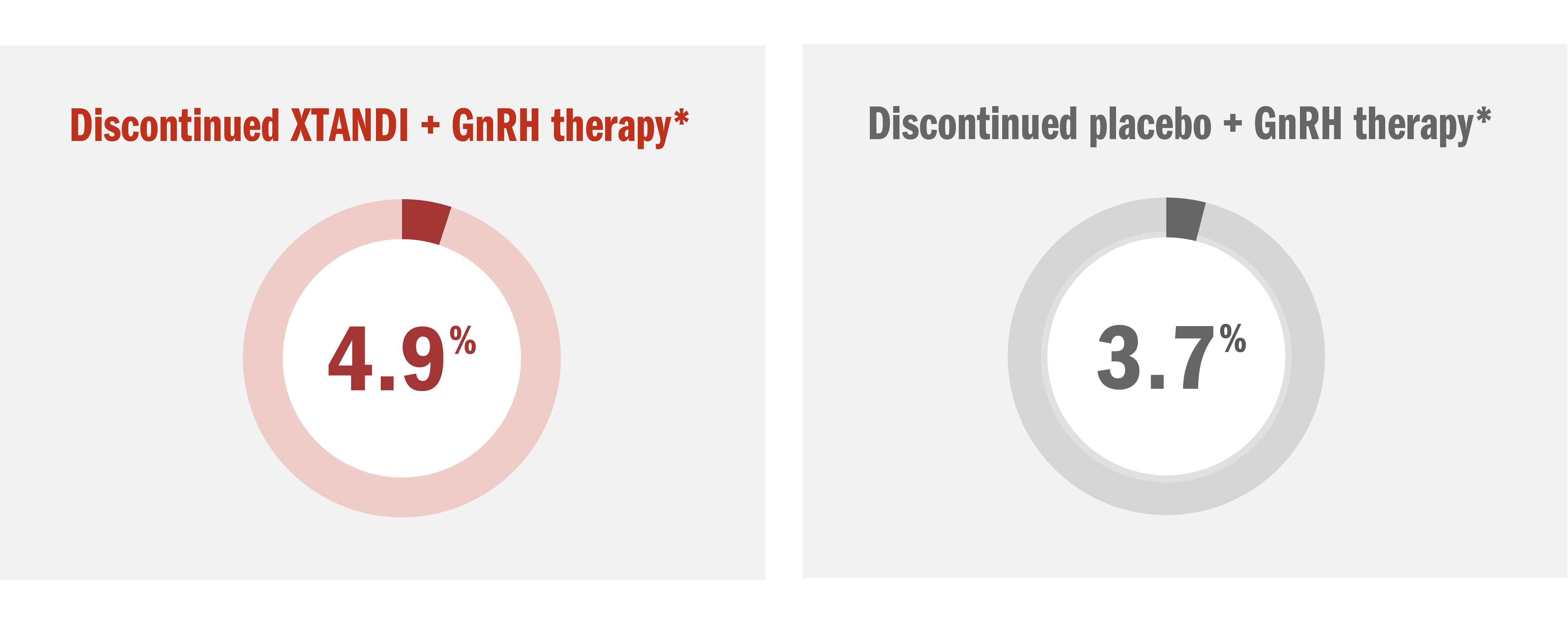
- The most common adverse reactions resulting in permanent discontinuation were alanine aminotransferase increased, aspartate aminotransferase elevation, and seizure, each in 0.3% of XTANDI + GnRH therapy*–treated patients
*Or after bilateral orchiectomy.1
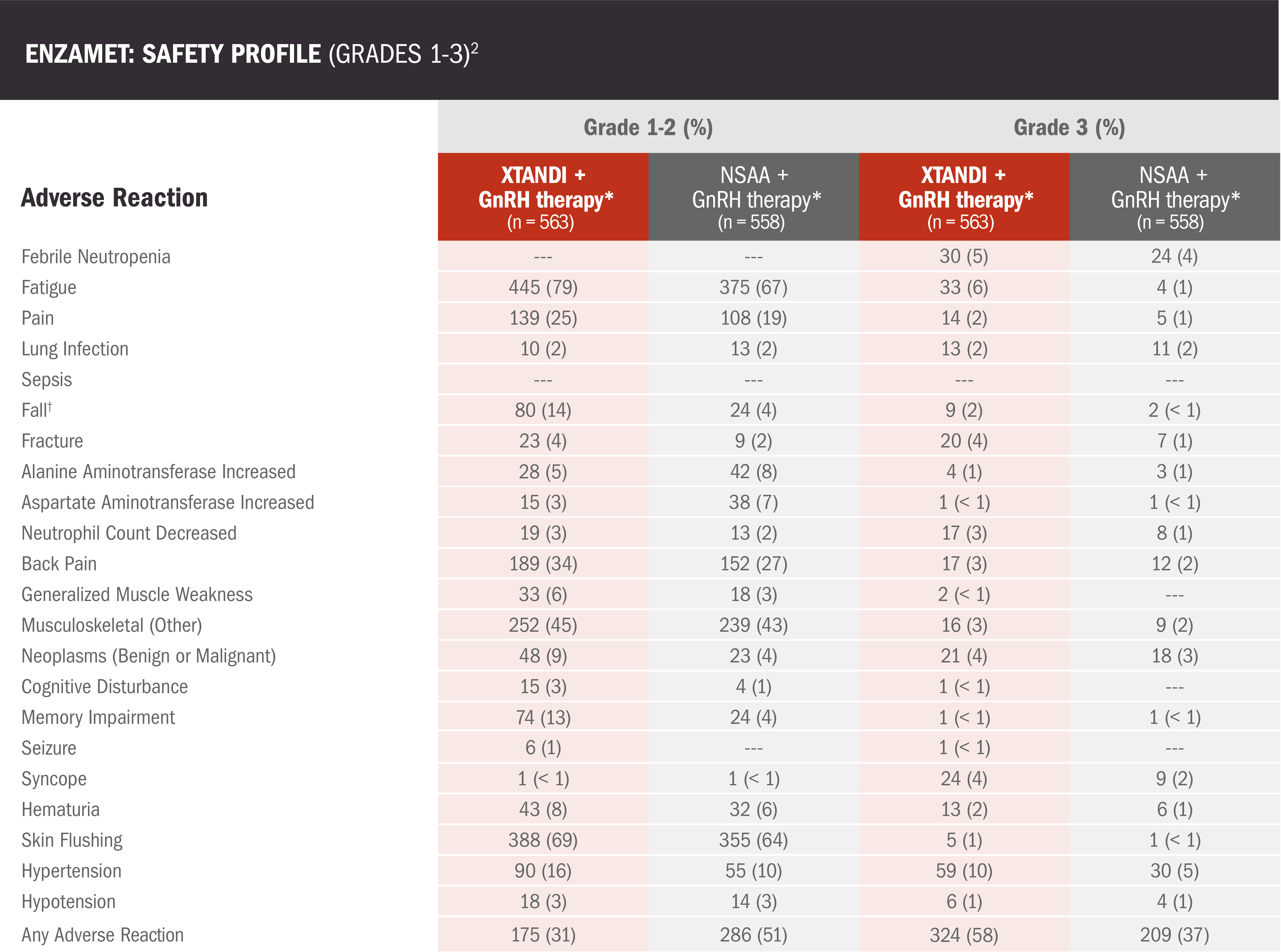
The safety profile of XTANDI in mCSPC was evaluated in the ENZAMET trial2
ENZAMET results were not accepted by the FDA for inclusion in the US Full Prescribing Information for XTANDI. The overall patient population in ENZAMET included patients who received or did not receive concomitant docetaxel. The efficacy and safety of XTANDI in combination with docetaxel has not been established.
- Among the patients who received concomitant docetaxel treatment, the addition of XTANDI was associated with additional adverse reactions2
Data are n (%) shown for grade 3 adverse reactions occurring in >2% of participants and other relevant events associated with XTANDI.2
- Among the patients who received concomitant docetaxel treatment, the addition of XTANDI was associated with additional adverse reactions2
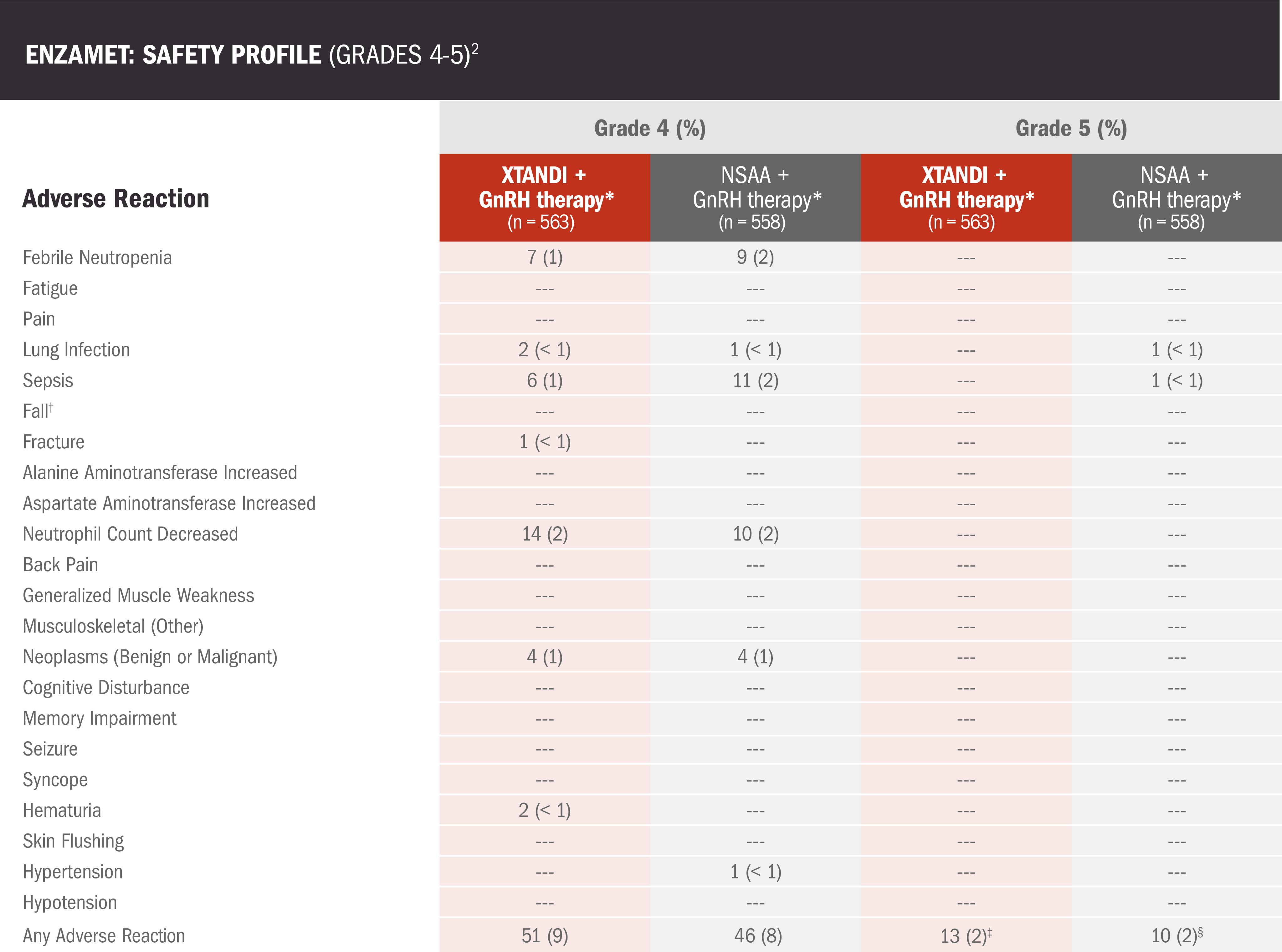
Data are n (%) shown for grade 4 adverse reactions occurring in >2% of participants and all grade 5 adverse reactions, plus other relevant events associated with XTANDI.2
*Or after bilateral orchiectomy.3
†The falls could be from balance problems, syncope, muscle weakness, or sarcopenia.2
‡Deaths reported as one cardiac disorder, two myocardial infarctions, three not specified, one general disorder, one sudden death, one acidosis, two strokes, one respiratory failure, and one respiratory disorder2
§Deaths reported as one cardiac arrest, one gastric hemorrhage, one gastrointestinal, one general disorder, one sudden death, four infections, and one pneumonitis.2
Safety profile in patients with nmCSPC with high-risk BCR1
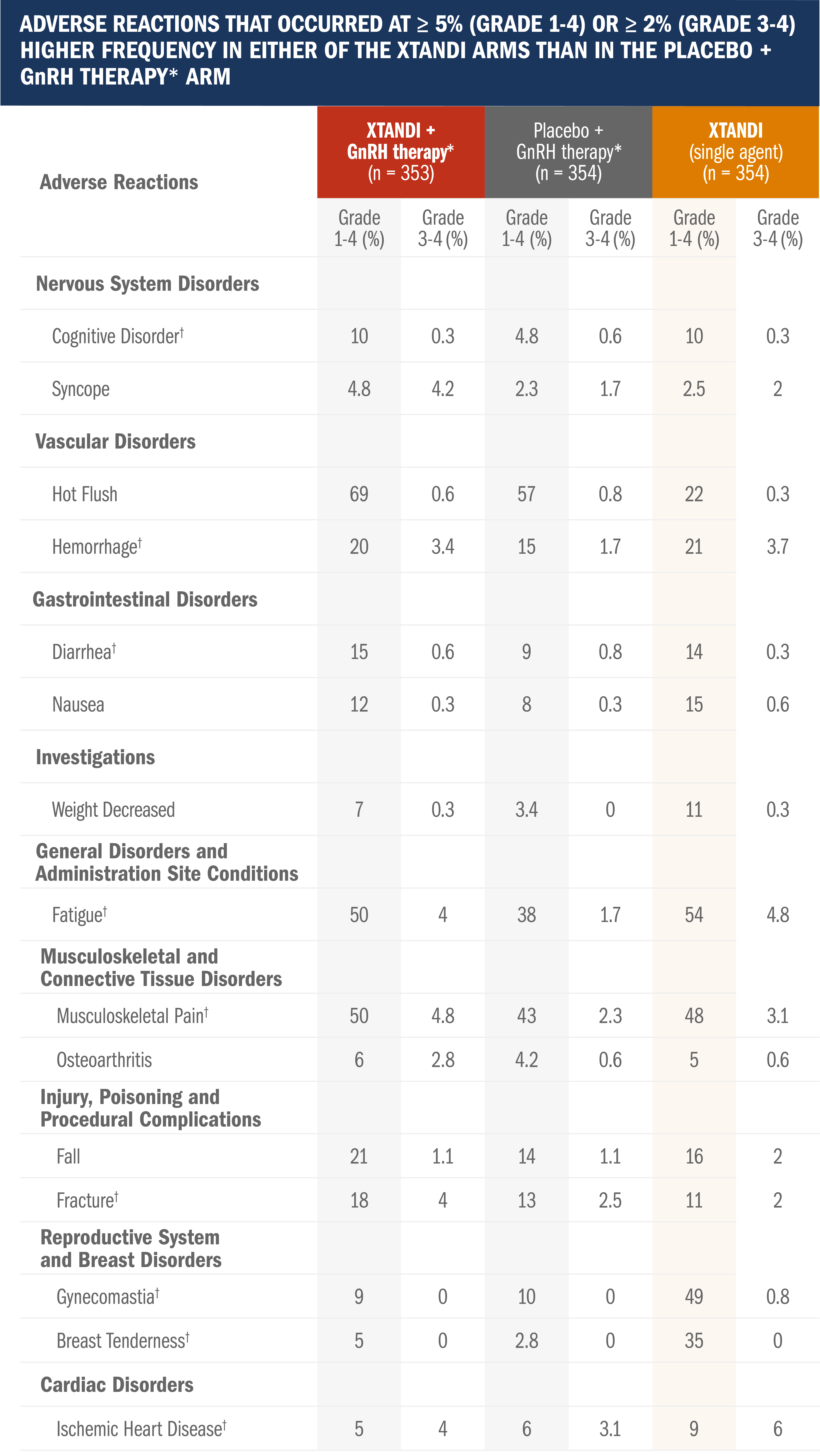
Adverse reactions were graded according to the CTCAE version 4.03.
- Median total duration of treatment was 60.6 months (range: 0.1-90.4 months) with XTANDI + GnRH therapy,* 55.6 months (range: 0.7-94.1 months) with placebo + GnRH therapy,* and 60.4 months (range: 0.4-95.0) with XTANDI (single agent)
- Median duration of drug treatment was 32.4 months (range: 0.1-83.4 months) with XTANDI + GnRH therapy,* 35.4 months (range: 0.7-85.7 months) with placebo + GnRH therapy,* and 45.9 months (range: 0.4-88.9) with XTANDI (single agent)
- Grade 3 or higher adverse reactions during total duration of treatment were reported in 46% of patients treated with XTANDI + GnRH therapy,* 43% of patients receiving placebo + GnRH therapy,* and 50% of patients receiving XTANDI (single agent)
*Leuprolide.1
†Includes multiple terms.
BCR, biochemical recurrence; CTCAE, Common Terminology Criteria for Adverse Events; GnRH, gonadotropin-releasing hormone; mCSPC, metastatic castration-sensitive prostate cancer; NEC, not elsewhere classifiable; nmCSPC, nonmetastatic castration-sensitive prostate cancer; NSAA, nonsteroidal anti-androgen.
References: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025. 2. Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334. doi:10.1016/S1470-2045(23)00063-3. 3. Protocol for: Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835.
BCR, biochemical recurrence; CSPC, castration-resistant prostate cancer; CTCAE, Common Terminology Criteria for Adverse Events; GnRH, gonadotropin-releasing hormone; mCSPC, metastatic castration-sensitive prostate cancer; nmCSPC, nonmetastatic castration-sensitive prostate cancer.
Reference: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025.