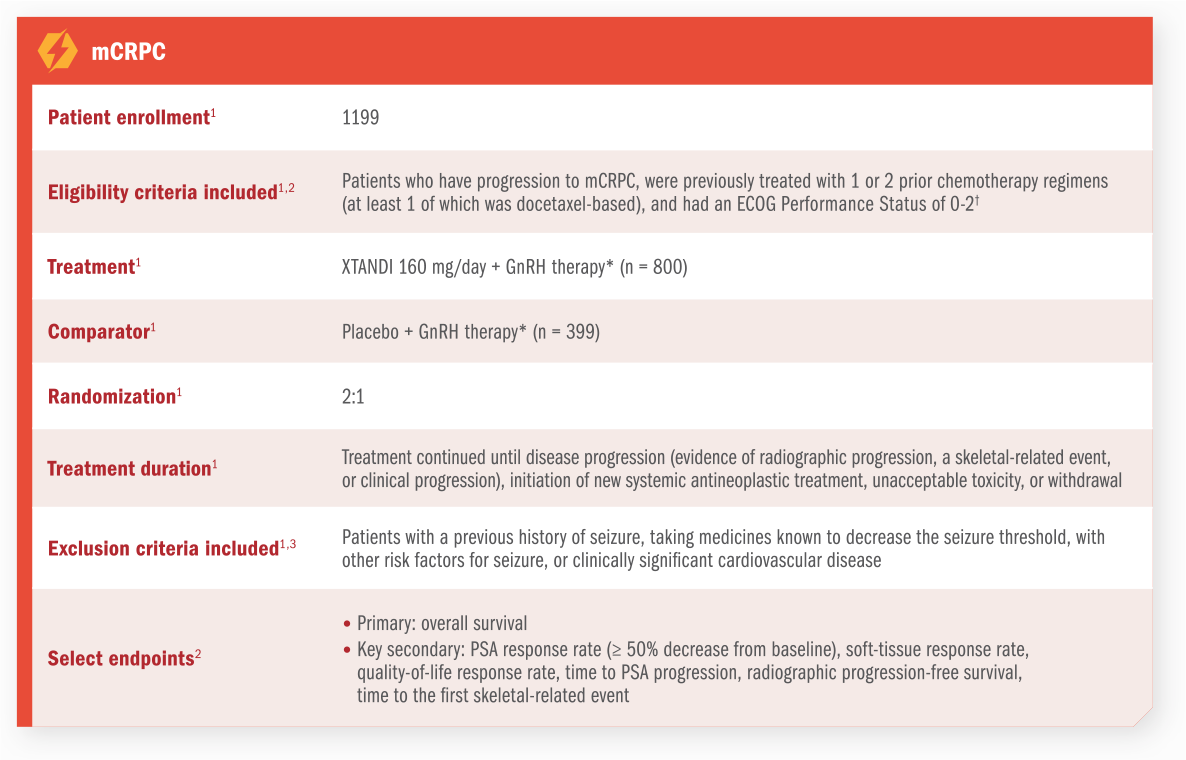
PREVAIL Trial
XTANDI + GnRH therapy* significantly extended overall survival in mCRPC1
PREVAIL was a multinational, double-blind, randomized, placebo-controlled phase 3 trial of XTANDI + GnRH therapy* in patients with mCRPC who were asymptomatic or mildly symptomatic.1,2
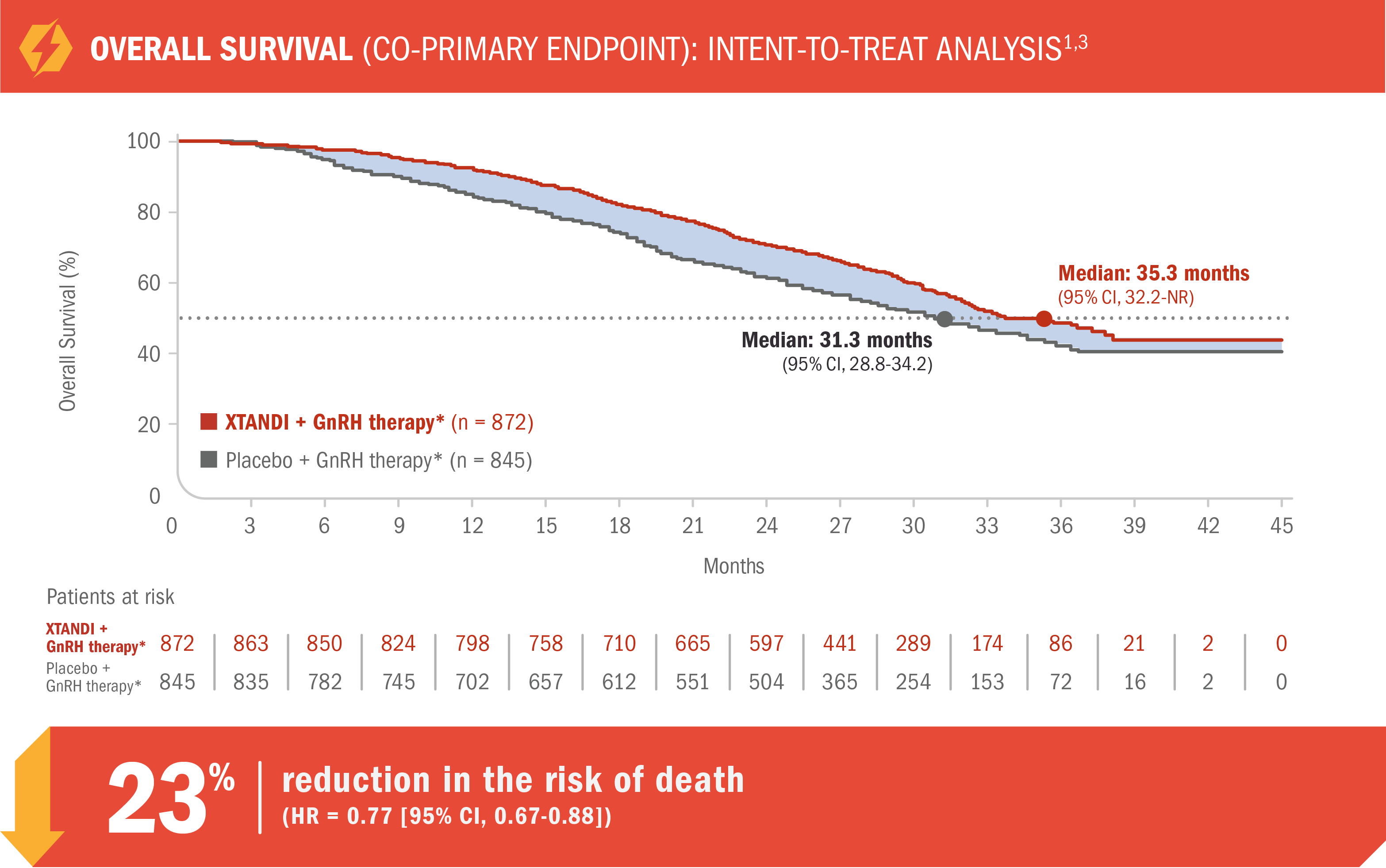
- Number of events: 368 (42%) with XTANDI + GnRH therapy* vs 416 (49%) with placebo + GnRH therapy*1
Median overall survival was 35.3 months (95% CI, 32.2-NR) for patients receiving XTANDI + GnRH therapy* vs 31.3 months (95% CI, 28.8-34.2) for those receiving placebo + GnRH therapy*1
- At a prespecified interim analysis for overall survival, there was a 29% reduction in the risk of death in patients who received XTANDI + GnRH therapy* vs placebo + GnRH therapy* (HR = 0.71 [95% CI, 0.60-0.84]; P < 0.0001)1
See mCRPC (PREVAIL) adverse reactions
*Or after bilateral orchiectomy.1
For patients on GnRH therapy* who have progressed to mCRPC1
PREVAIL was a multinational, double-blind, randomized, placebo-controlled phase 3 trial of XTANDI + GnRH therapy* in patients with mCRPC who were asymptomatic or mildly symptomatic1,2
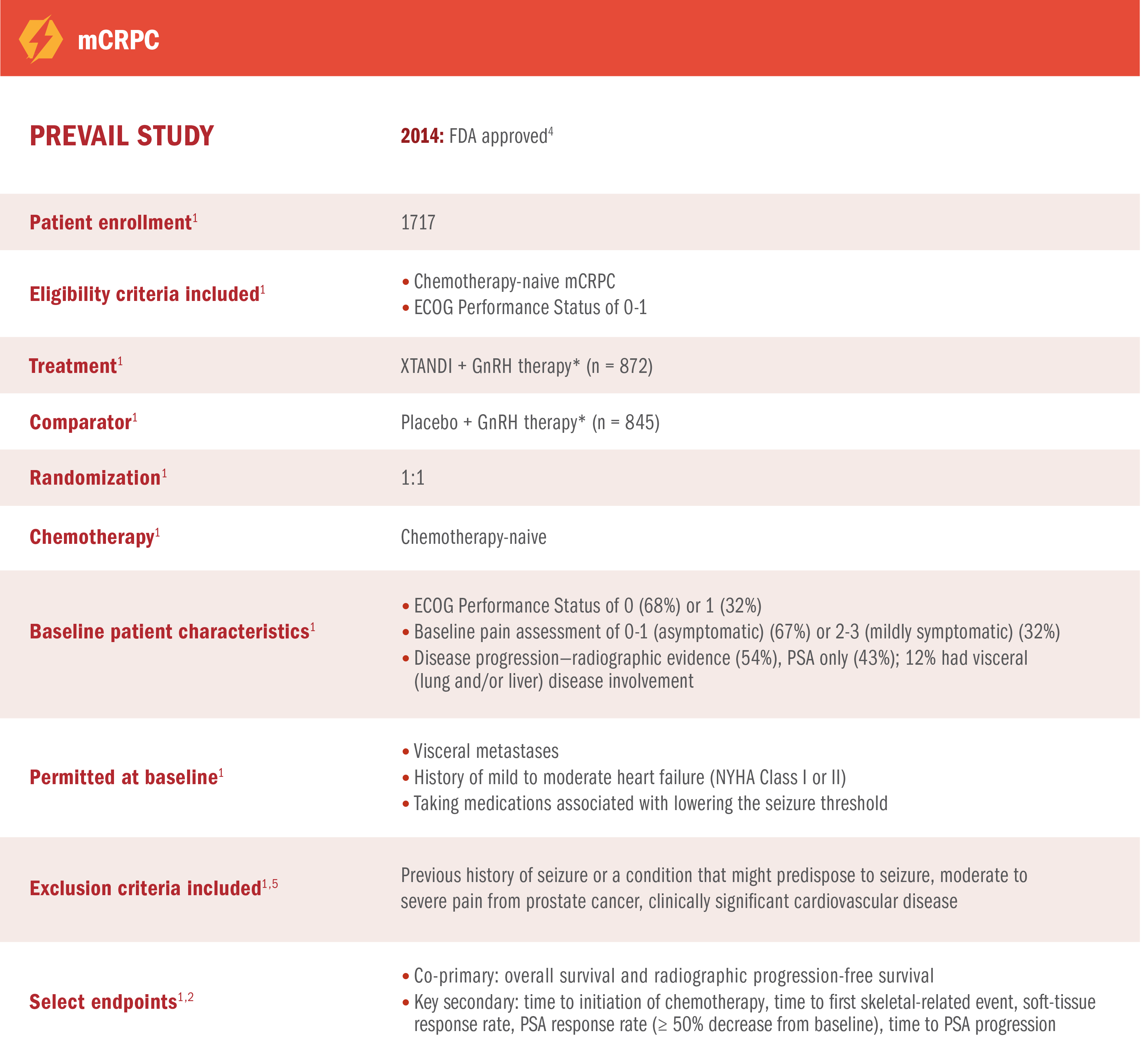
*Or after bilateral orchiectomy.1
XTANDI + GnRH therapy* significantly reduced the risk of radiographic disease progression† in patients with mCRPC1
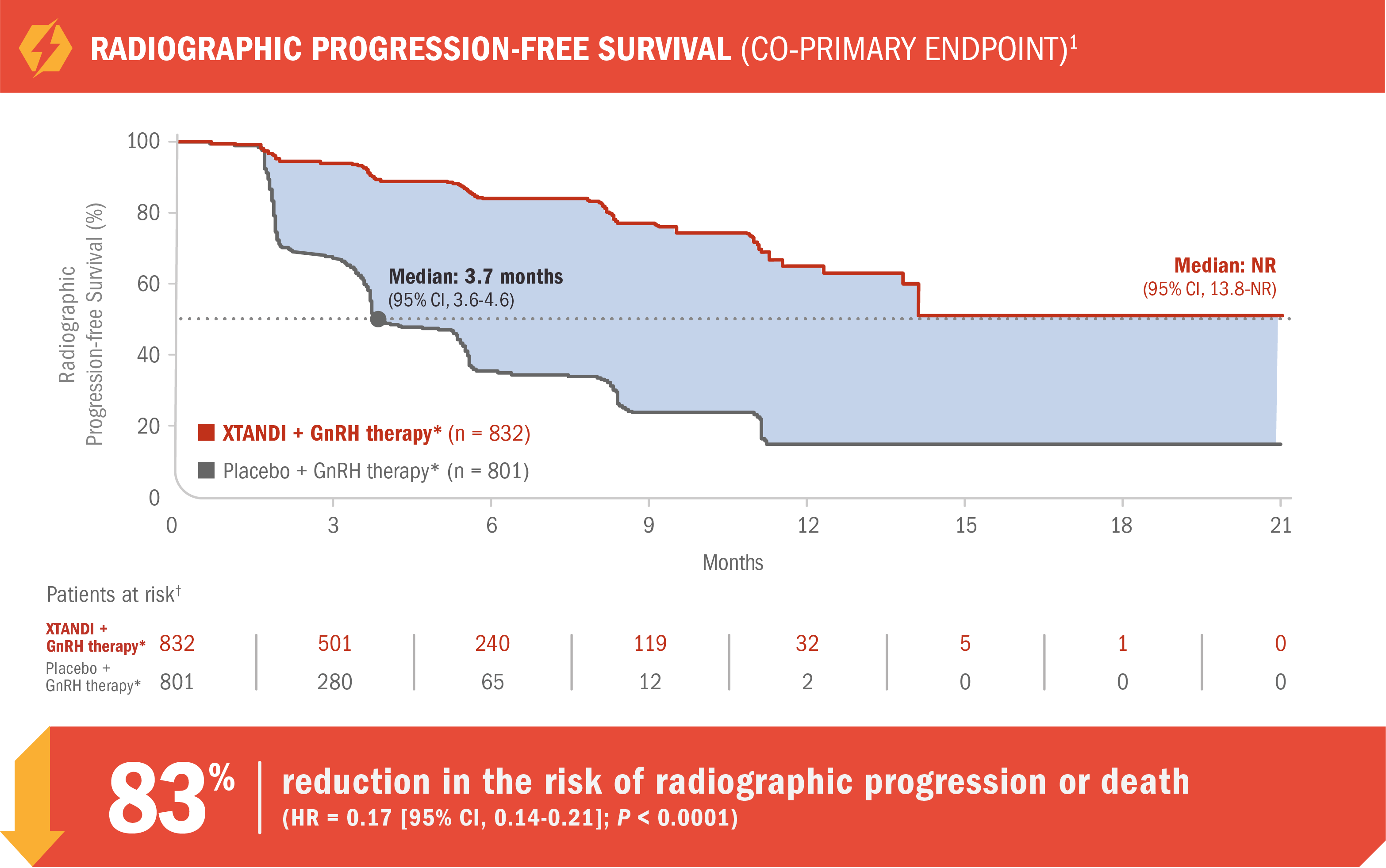
- Number of events: 118 (14%) with XTANDI + GnRH therapy* vs 320 (40%) with placebo + GnRH therapy*1
- Median radiographic progression-free survival was not reached (95% CI, 13.8-NR) with XTANDI + GnRH therapy* vs 3.7 months (95% CI, 3.6-4.6) with placebo + GnRH therapy*1
Radiographic progression was assessed with the use of sequential imaging and was defined by bone scan identification of 2 or more new bone lesions with confirmation (PCWG2) and/or RECIST 1.1 criteria for progression of soft-tissue lesions. The primary analysis of radiographic progression-free survival utilized centrally reviewed radiographic assessment of progression.1
Radiographic event was defined as first evidence of disease progression as assessed by independent investigators.1
*Or after bilateral orchiectomy.1
†The patients at risk are those trial subjects from whom radiographic progression-free survival is calculated at each time point.6
XTANDI + GnRH therapy* significantly delayed time to cytotoxic chemotherapy initiation1
17.2 additional months (median) until chemotherapy initiation with XTANDI + GnRH therapy* vs placebo + GnRH therapy*1
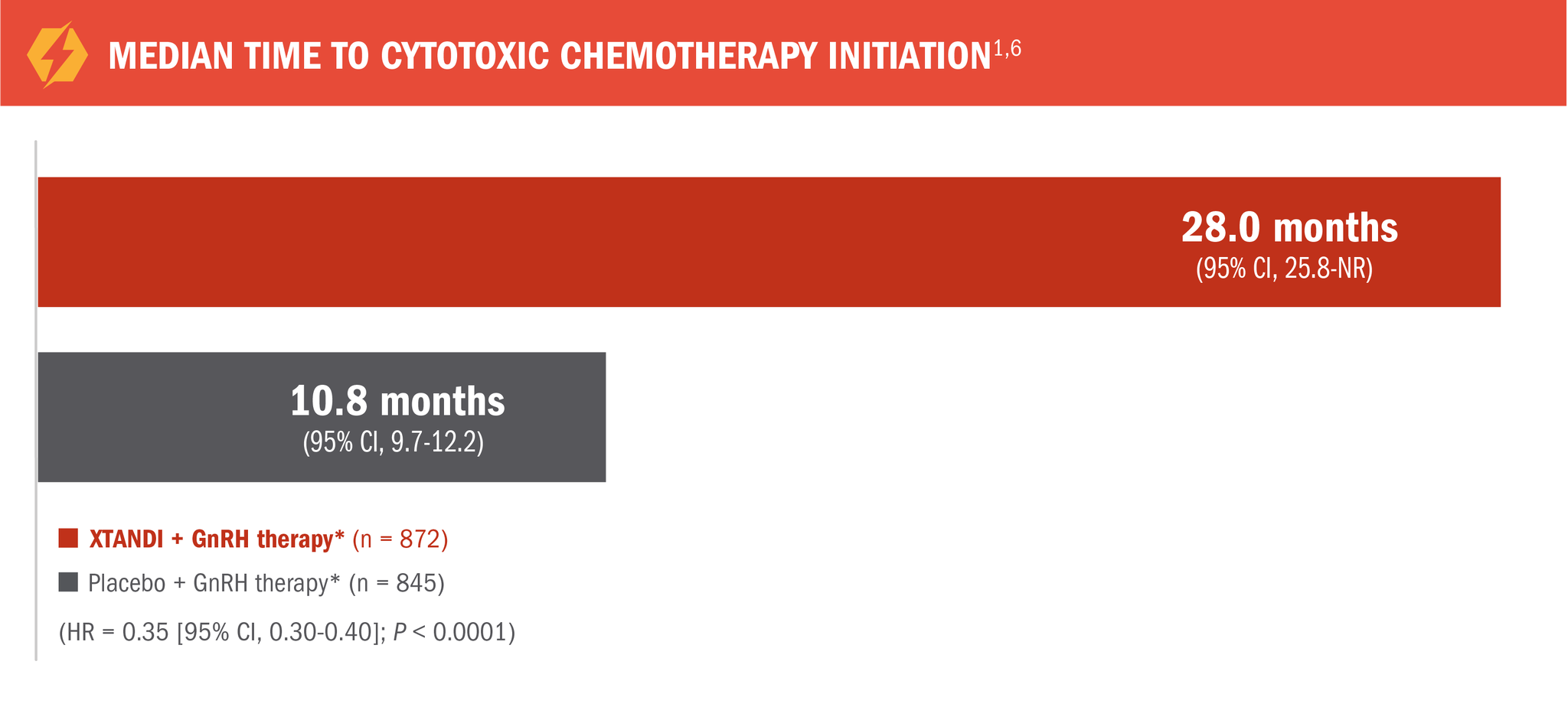
*Or after bilateral orchiectomy.1
XTANDI + GnRH therapy* significantly reduced the risk of first skeletal-related event†1

*Or after bilateral orchiectomy.1
†A skeletal-related event was defined as radiation therapy or surgery to bone for prostate cancer, pathologic bone fracture, spinal cord compression, or change of antineoplastic therapy to treat bone pain.1
PREVAIL: Docetaxel and abiraterone acetate were the most frequently prescribed therapies following disease progression on XTANDI + GnRH therapy*7

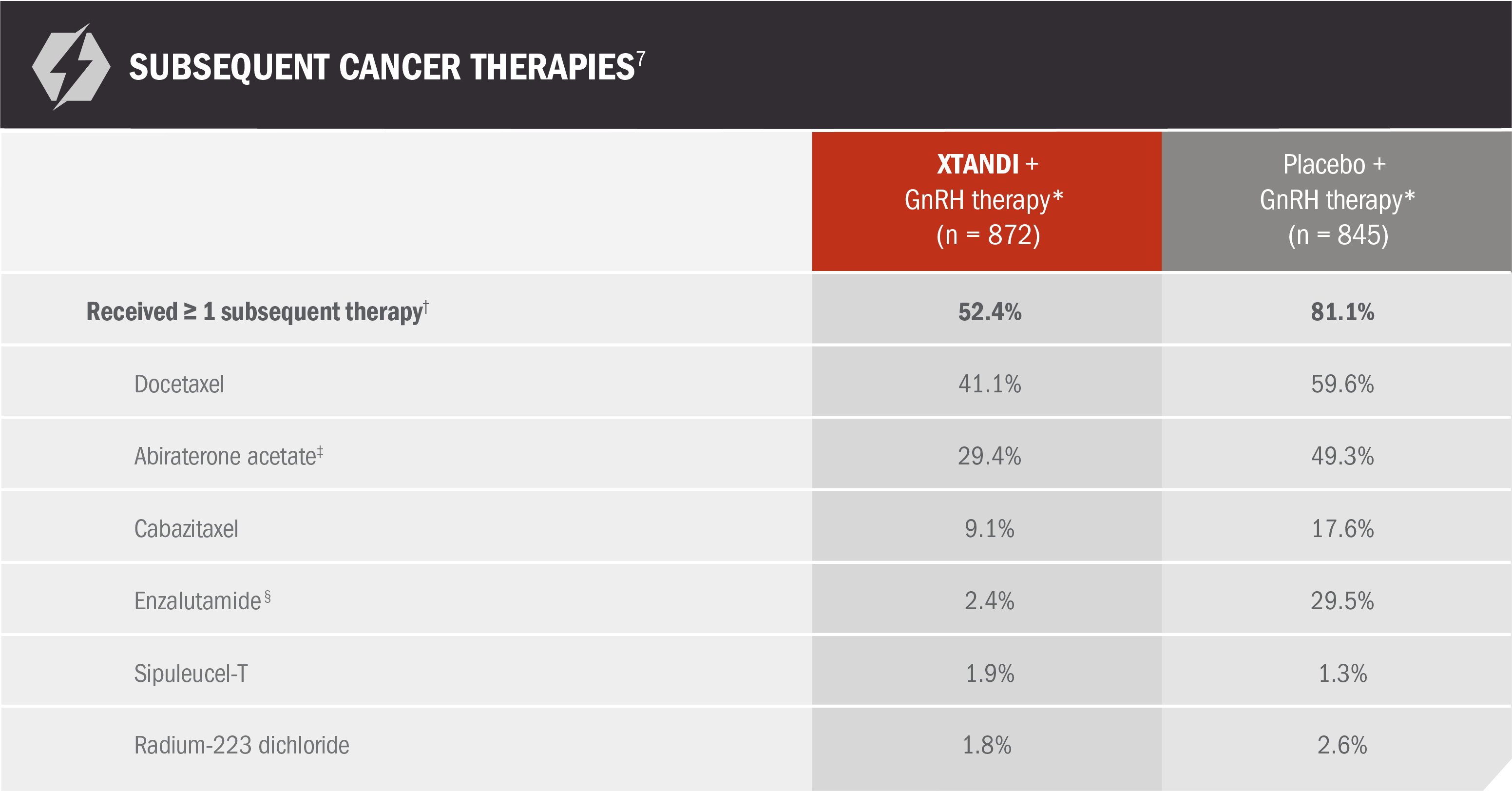
Use of subsequent therapies occurred after disease progression but during the evaluation period for overall survival7
*Or after bilateral orchiectomy.1
†Patients taking any of the postbaseline antineoplastic therapies that have demonstrated overall survival benefit in clinical trials.7
‡Concomitant use was allowed before study drug discontinuation in patients with confirmed radiographic progression or a skeletal-related event.7
§Patients assigned to the placebo group who received enzalutamide in the open-label period are included in the placebo column for subsequent enzalutamide therapy.7
XTANDI in mCRPC
(PREVAIL TRIAL
PUBLICATION)
XTANDI in mCRPC
(PREVAIL TRIAL PUBLICATION)
Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151-154. doi:10/1016/j.eururo.2016.07.032.
View The PublicationXTANDI + GnRH therapy* reduced the risk of radiographic progression or death vs bicalutamide in patients with mCRPC1
TERRAIN was a multinational, double-blind, randomized, phase 2 trial comparing XTANDI + GnRH therapy* with bicalutamide + GnRH therapy* in mCRPC patients who were asymptomatic or mildly symptomatic and had not received prior chemotherapy.1,8
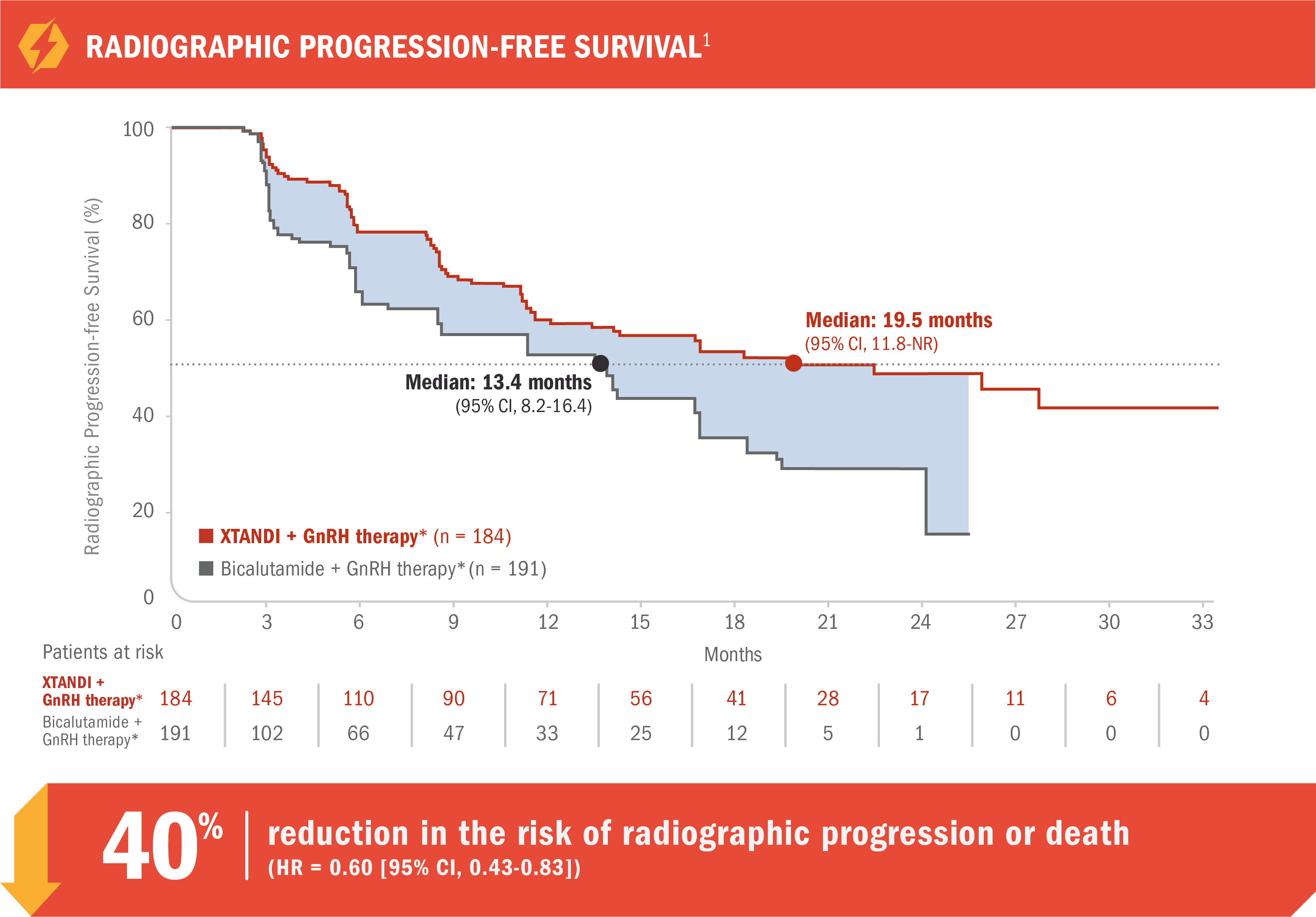
- Number of events: 72 (39%) with XTANDI + GnRH therapy* vs 74 (39%) with bicalutamide + GnRH therapy*1
Radiographic progression-free survival was defined as the time from randomization to the first objective evidence of radiographic progression as assessed by ICR or death, whichever occurred first.1
Radiographic progression was assessed by ICR per PCWG2 criteria and/or RECIST 1.1 criteria for progression of bone and soft-tissue lesions, respectively.1,9
- Median radiographic progression-free survival was 19.5 months (95% CI, 11.8-NR) for patients receiving XTANDI + GnRH therapy* vs 13.4 months (95% CI, 8.2-16.4) for patients receiving bicalutamide + GnRH therapy* (HR = 0.60 [95% CI, 0.43-0.83])1
See mCRPC (TERRAIN) adverse reactions
*Or after bilateral orchiectomy.1
For patients on GnRH therapy* who have progressed to mCRPC†1
TERRAIN was a multinational, double-blind, randomized, phase 2 trial comparing XTANDI + GnRH therapy* with bicalutamide + GnRH therapy* in mCRPC patients who were asymptomatic or mildly symptomatic and had not received prior chemotherapy1,8
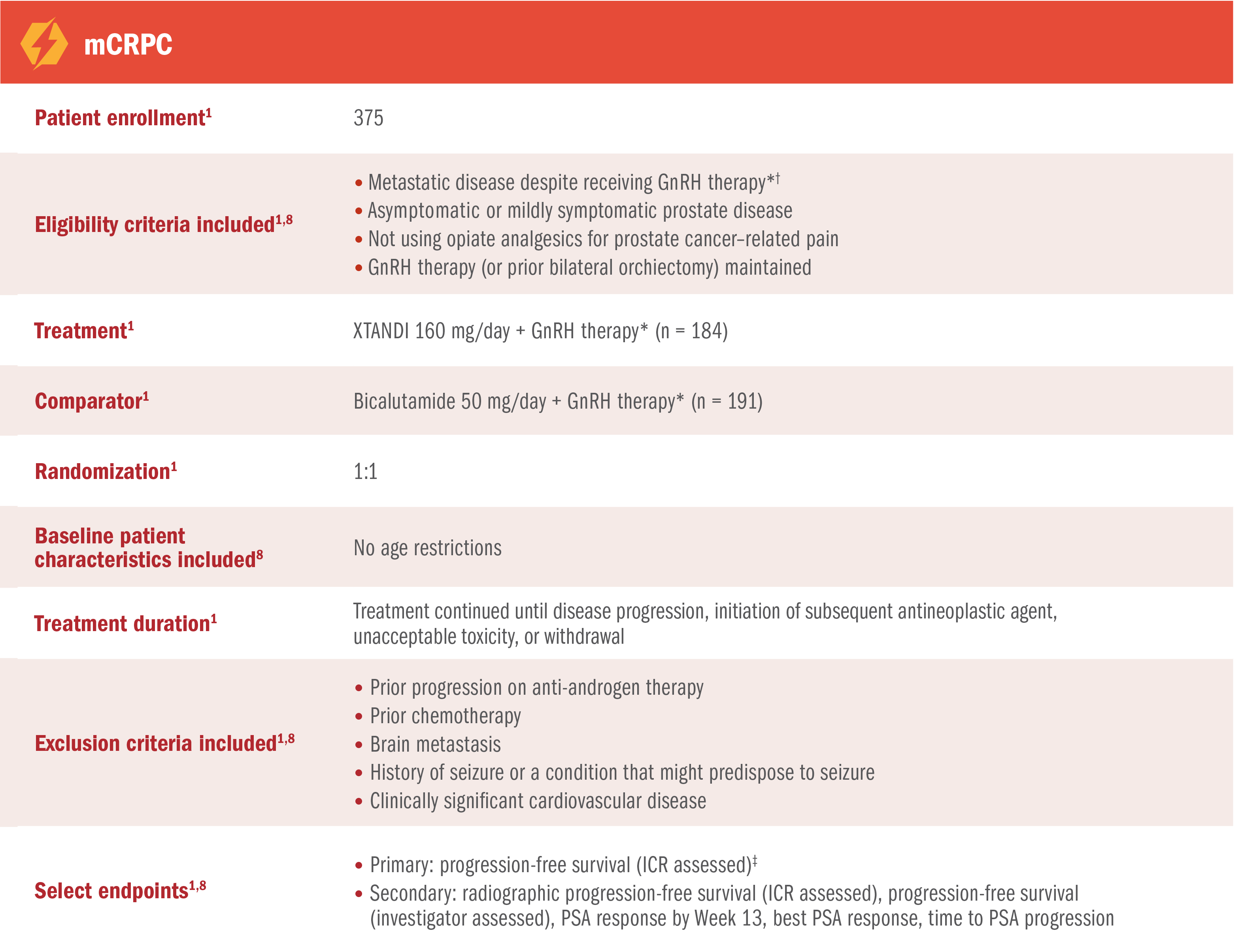
The US Full Prescribing Information for XTANDI includes a modified efficacy analysis of the radiographic progression-free survival§ endpoint per PCWG2 criteria.1
*Or after bilateral orchiectomy.1
†Disease progression was defined as the presence of 1 or more of the following 3 criteria: 1) PSA progression (≥ 3 measurements of rising PSA concentrations with an interval of at least 1 week between determinations); 2) soft-tissue disease progression defined by RECIST 1.1; 3) bone disease progression defined by at least 2 new lesions on bone scan.8
‡Progression-free survival was defined as the time from randomization to the first progression event, which includes radiographic disease progression, skeletal-related event, initiation of a new antineoplastic therapy, and death.8
§Radiographic progression-free survival was defined as the time from randomization to the first objective evidence of radiographic progression as assessed by ICR or death, whichever occurred first. Radiographic progression was assessed by ICR per PCWG2 criteria and/or RECIST 1.1 criteria for progression of bone and soft-tissue lesions, respectively.1,8
Progression-free survival of XTANDI and bicalutamide was also assessed in patients with mCRPC8
The following results are from the TERRAIN trial that contributed to the clinical body of evidence for XTANDI but are not contained in the US Full Prescribing Information for XTANDI. The US Full Prescribing Information for XTANDI only includes a modified efficacy analysis of the radiographic progression-free survival endpoint, as shown above.
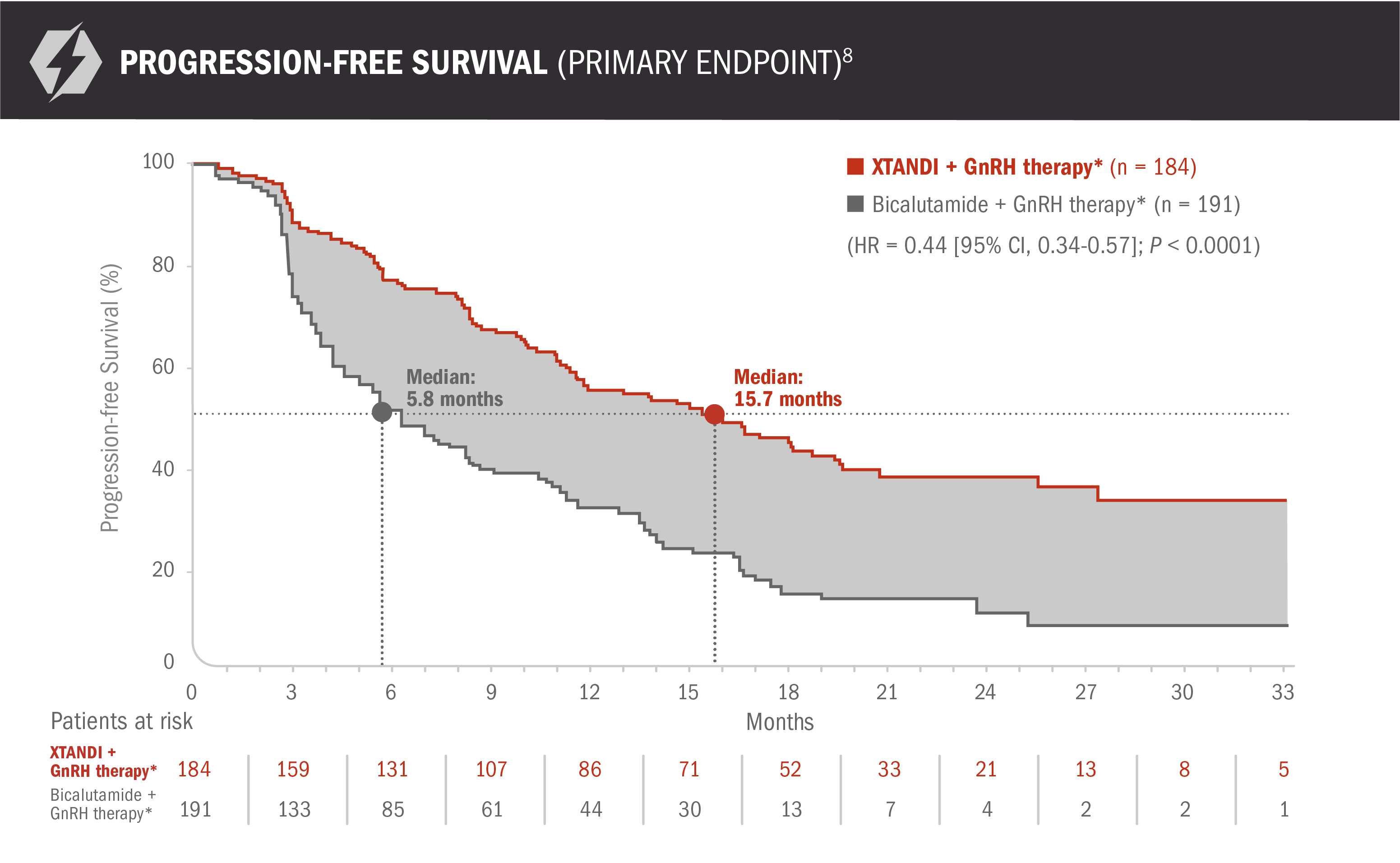
- XTANDI + GnRH therapy* improved progression-free survival, with a 56% reduction in risk of progression event, including death, vs bicalutamide + GnRH therapy* (HR = 0.44 [95% CI, 0.34-0.57]; P < 0.0001)8
- Number of events: 99 (54%) with XTANDI + GnRH therapy* and 141 (74%) with bicalutamide + GnRH therapy* - Median progression-free survival was 15.7 months (95% CI, 11.5-19.4) for patients receiving XTANDI + GnRH therapy* and 5.8 months (95% CI, 4.8-8.1) for patients receiving bicalutamide + GnRH therapy*8
Progression-free survival was a composite endpoint defined as the time from randomization to the first progression event, which included 4 components: (1) radiographic disease progression, (2) initiation of a new antineoplastic therapy, (3) skeletal-related event, or (4) death.8,9 Two of the 4 progression-free survival components as measured in the study (radiographic disease progression and initiation of a new antineoplastic therapy) have limitations that may impact the reliability and objectivity of the progression-free survival endpoint to measure the treatment effects between study drugs:
- “Radiographic disease progression” criteria were different than the standard PCWG2 criteria, which may have led to inflation of the treatment effect of XTANDI. In the US Full Prescribing Information for XTANDI, radiographic progression-free survival was redefined to maintain consistency with PCWG21,8,10
- “Initiation of a new antineoplastic therapy” was not standardized, and the clinical decision on when to initiate a new therapy may have introduced bias1,6,8
*Or after bilateral orchiectomy.1
PSA changes vs bicalutamide in patients with mCRPC8
PSA data are not reported in the US Full Prescribing Information for XTANDI because PSA is not a reliable surrogate for overall survival. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.
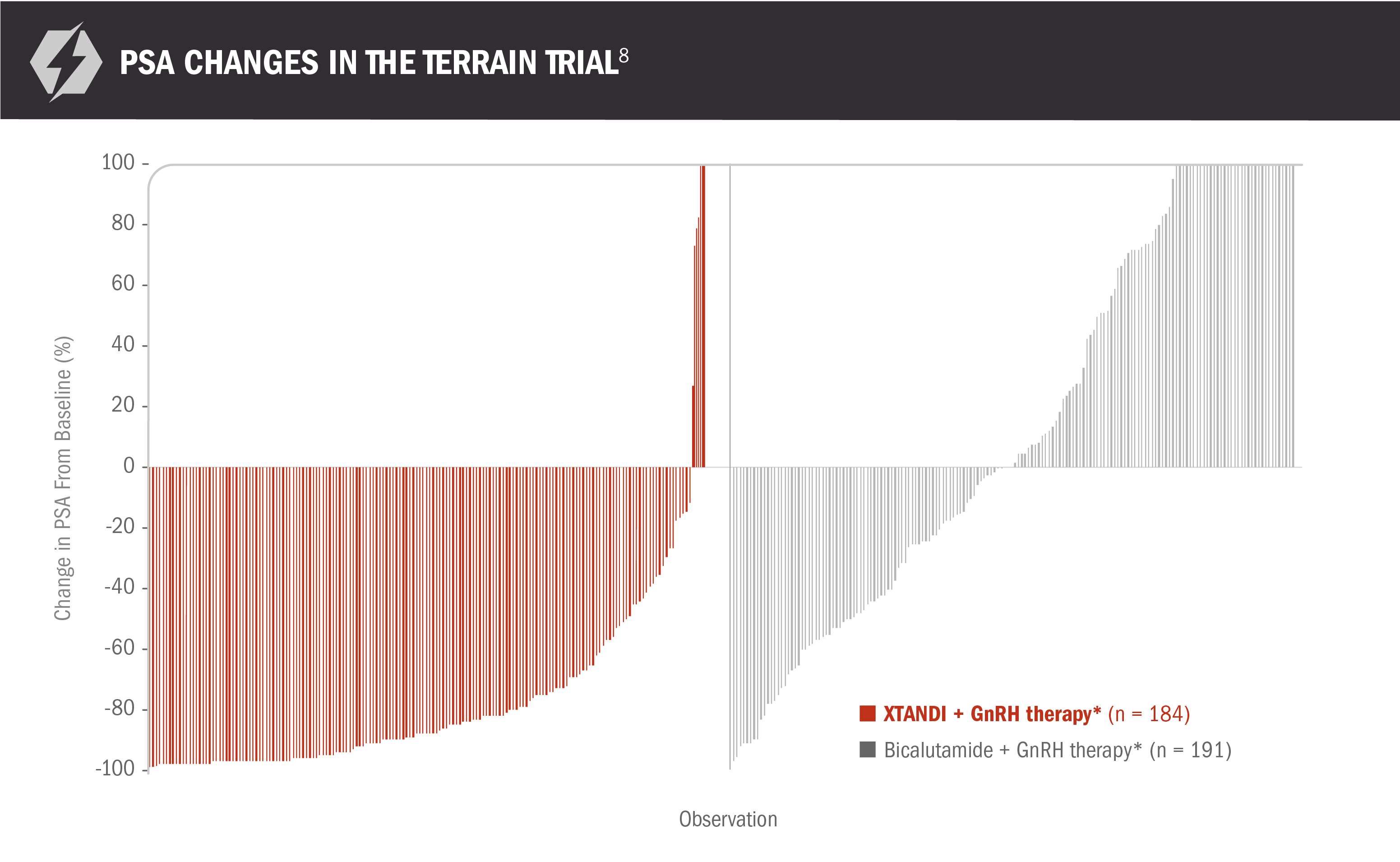
- Median time to a PSA progression event was 19.4 months (95% CI, 16.6-NR) for patients receiving XTANDI + GnRH therapy* and 5.8 months (95% CI, 5.6-8.3) for patients receiving bicalutamide + GnRH therapy*8
- Reductions in PSA of ≥ 50% were observed in 82% (151 of 184) of patients receiving XTANDI + GnRH therapy* and 21% (40 of 191) of patients receiving bicalutamide + GnRH therapy*8
*Or after bilateral orchiectomy.1
XTANDI in mCRPC
(TERRAIN TRIAL
PUBLICATION)
XTANDI in mCRPC
(TERRAIN TRIAL PUBLICATION)
Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. doi:10.1016/S1470-2045(15)00518-5.
View The PublicationXTANDI + GnRH therapy* extended median overall survival post-docetaxel in patients with mCRPC1
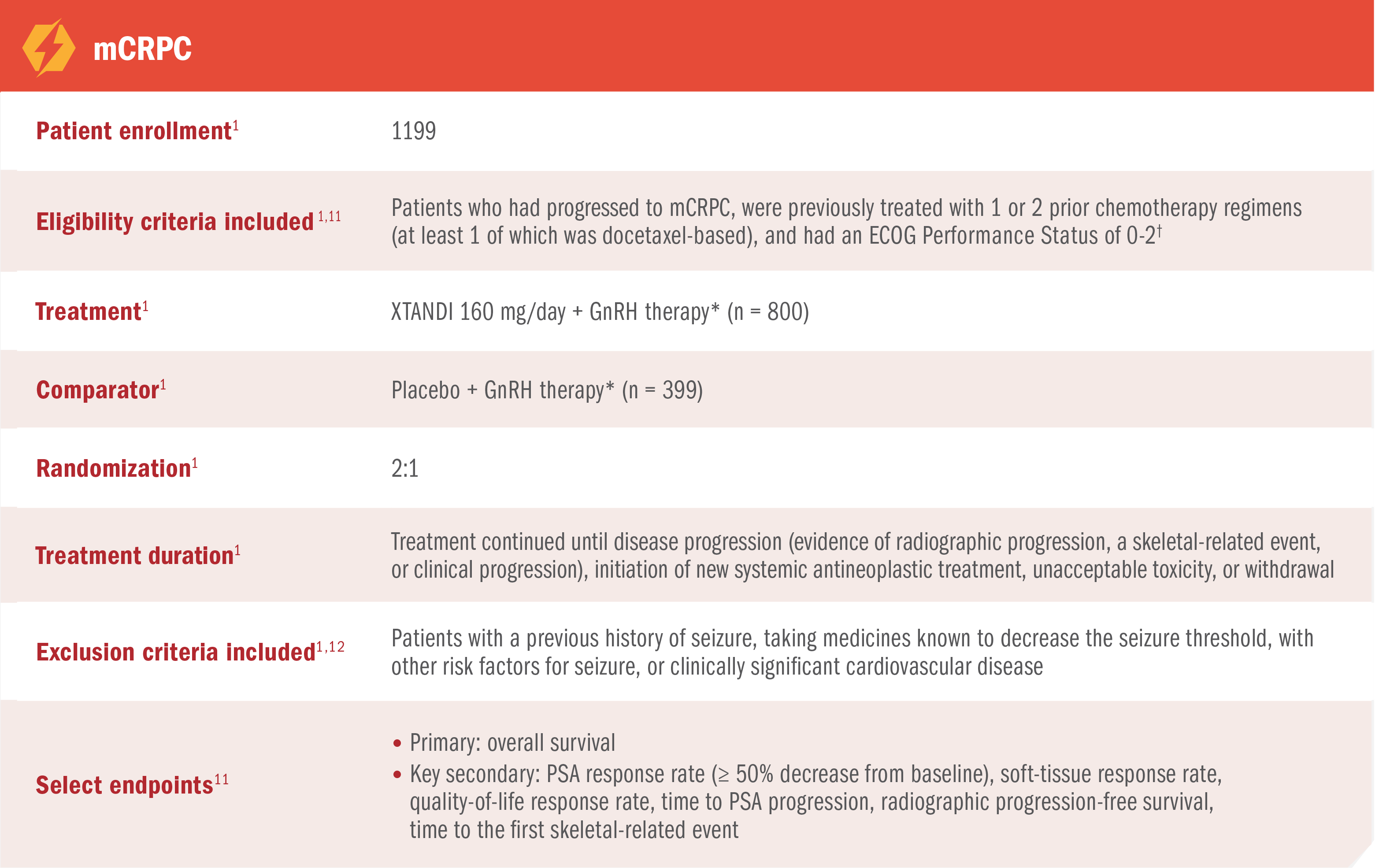
AFFIRM was a multinational, randomized, double-blind, placebo-controlled phase 3 trial of XTANDI + GnRH therapy* vs placebo + GnRH therapy* in patients who had received prior docetaxel-based chemotherapy.1,11
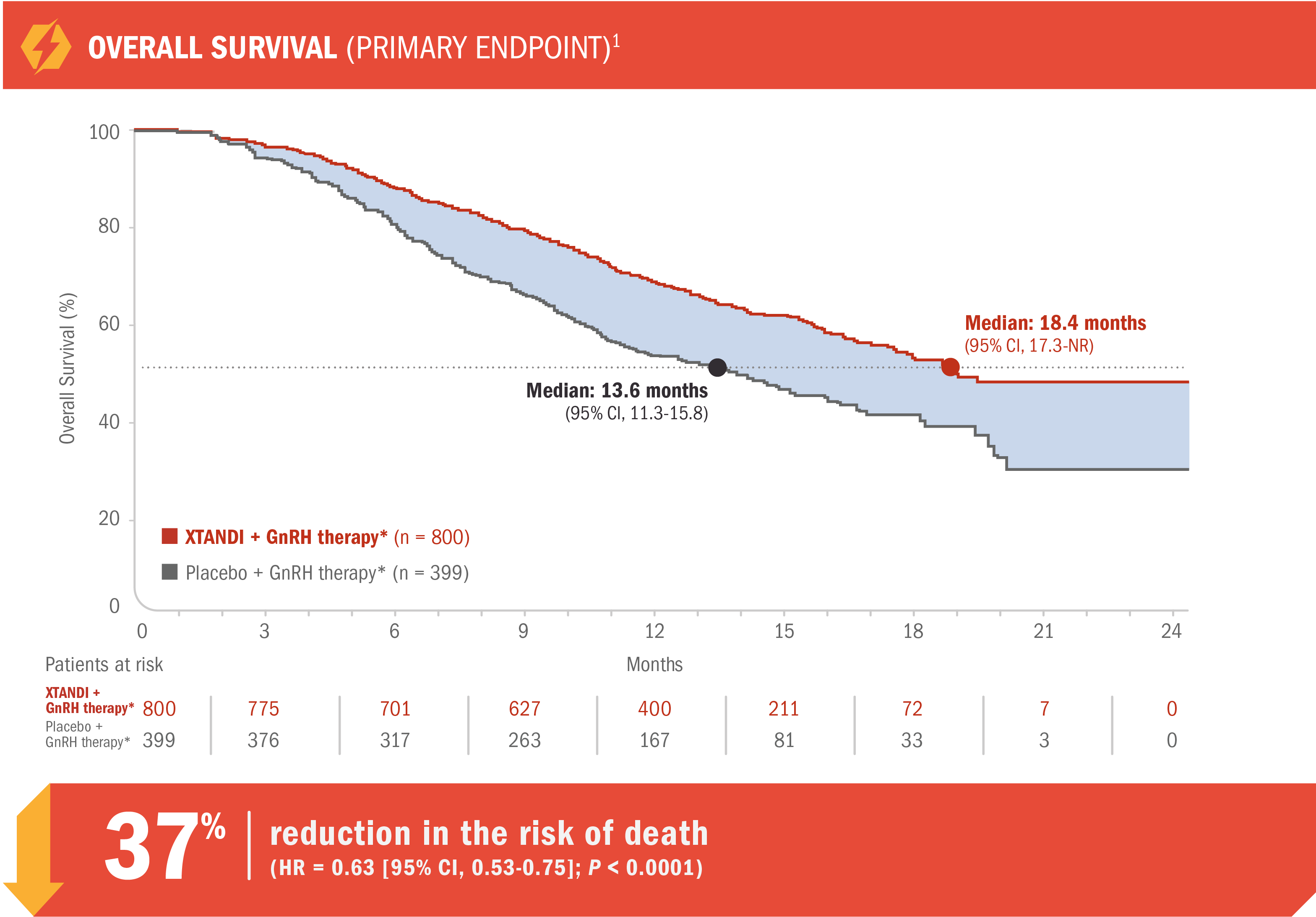
- 18.4 months (95% CI, 17.3-NR) median overall survival for patients receiving XTANDI + GnRH therapy* vs 13.6 months (95% CI, 11.3-15.8) for those receiving placebo + GnRH therapy*1
- Number of events: 308 (38.5%) with XTANDI + GnRH therapy* vs 212 (53.1%) with placebo + GnRH therapy*1
- Treatment was continued until disease progression,† initiation of a new systemic antineoplastic treatment, unacceptable toxicity, or study withdrawal. Progression was first assessed at 13 weeks1,11
See mCRPC (AFFIRM) adverse reactions
*Or after bilateral orchiectomy.1
†Disease progression was defined as radiographic progression, a skeletal-related event, or clinical progression. Radiographic progression of soft-tissue disease was defined according to RECIST 1.1. Radiographic progression of osseous disease was defined according to bone scans showing 2 or more new lesions. A skeletal-related event was defined as radiation therapy or surgery to bone, pathologic bone fracture, spinal cord compression, or change in antineoplastic therapy to treat bone pain.11
For patients on GnRH therapy* who have progressed to mCRPC1
AFFIRM was a multinational, randomized, double-blind, placebo-controlled phase 3 trial of XTANDI + GnRH therapy* vs placebo + GnRH therapy* in patients who had received prior docetaxel-based chemotherapy1,11
*Or after bilateral orchiectomy.1
†Ninety-two percent of study participants had an ECOG Performance Status score of 0 or 1. ECOG Performance Status scores range from 0 to 5, with 0 indicating full activity and 1 indicating a restriction in strenuous activity but the ability to be ambulatory and do light work.1,11
XTANDI in mCRPC
(Affirm TRIAL
PUBLICATION)
XTANDI in mCRPC
(Affirm TRIAL PUBLICATION)
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi:10.1056/NEJMoa1207506.
View The PublicationPROSPER Trial
XTANDI + GnRH therapy* significantly improved metastasis-free survival† vs placebo + GnRH therapy*1
PROSPER was a multinational, randomized, double-blind, placebo-controlled, phase 3 study in patients with nmCRPC who had progressed‡ on GnRH therapy.*1,2
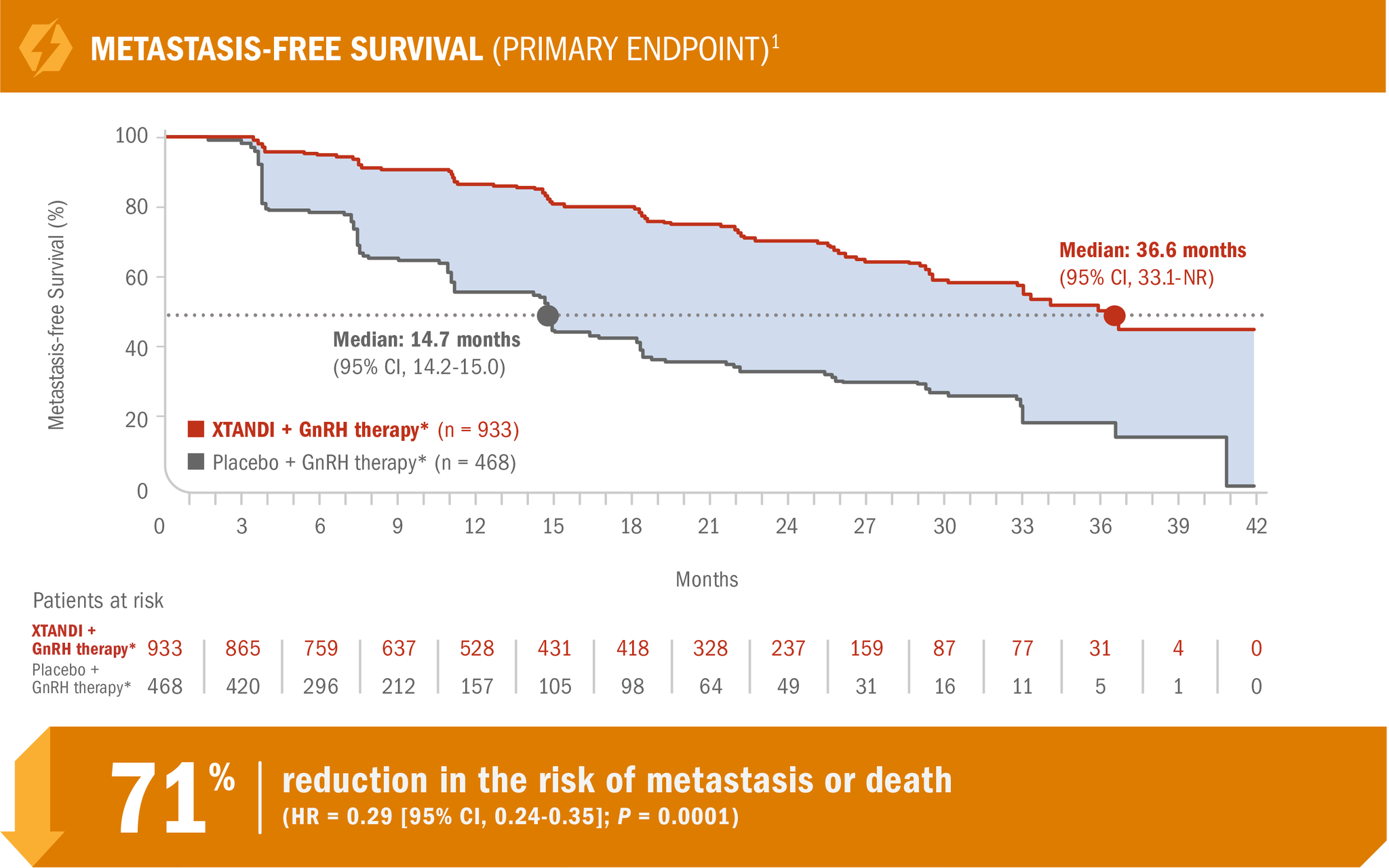
- Number of events: 219 (23.5%) with XTANDI + GnRH therapy* vs 228 (48.7%) with placebo + GnRH therapy*1
XTANDI + GnRH therapy* demonstrated consistent metastasis-free survival results in prespecified and stratified patient subgroups of PSA doubling time (< 6 months or ≥ 6 months) and use of a prior bone-targeting agent (yes or no).1
See nmCRPC (PROSPER) adverse reactions
*Or after bilateral orchiectomy.1
†The primary endpoint of the study was metastasis-free survival, defined as the time from randomization to whichever of the following occurred first: 1) loco-regional and/or distant radiographic progression per BICR; or 2) death up to 112 days after treatment discontinuation without evidence of radiographic progression.1
‡Progression was defined as at least 3 rising PSA values (PSA1 < PSA2 < PSA3) taken at least 1 week apart despite castrate levels of testosterone (≤ 50 ng/dL) on GnRH therapy or after bilateral orchiectomy.2
PROSPER was a multinational, randomized, double-blind, placebo-controlled, phase 3 study in patients with nmCRPC who had progressed* on GnRH therapy†1,2
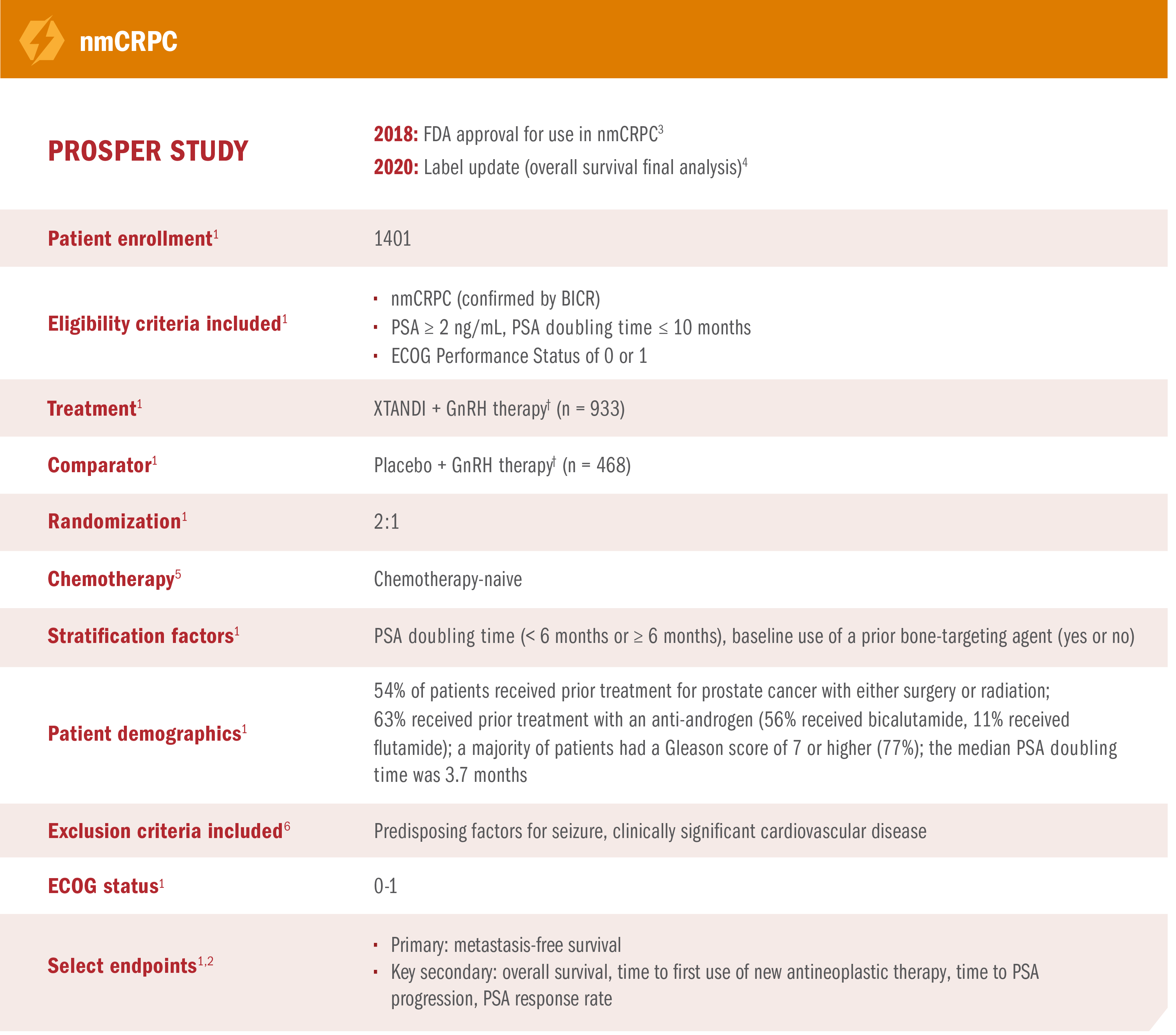
*Progression was defined as at least 3 rising PSA values (PSA1 < PSA2 < PSA3) taken at least 1 week apart despite castrate levels of testosterone (≤50 ng/dL) on GnRH therapy or after bilateral orchiectomy.2
†Or after bilateral orchiectomy.1
XTANDI + GnRH therapy* significantly extended overall survival† in patients with nmCRPC1
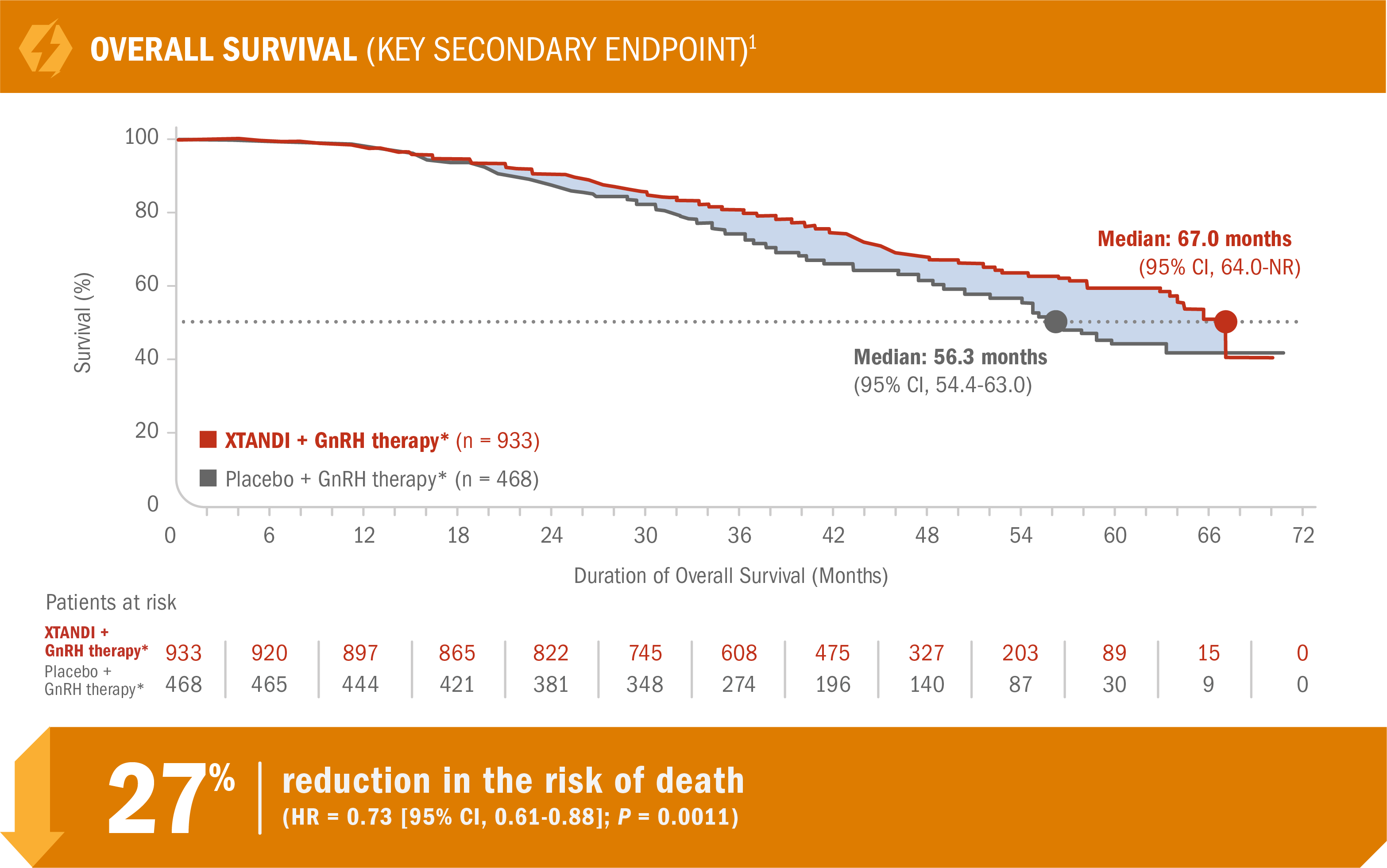
- Number of events: 288 (30.9%) with XTANDI + GnRH therapy* vs 178 (38.0%) with placebo + GnRH therapy*1
HR is based on a Cox regression model (with treatment as the only covariate) stratified by PSA doubling time and prior or concurrent use of a bone-targeting agent. The HR is relative to placebo with < 1 favoring XTANDI.7
The P value is based on a stratified log-rank test by PSA doubling time (< 6 months or ≥ 6 months) and prior or concurrent use of a bone-targeting agent (yes, no).1
The prespecified final analysis of overall survival occurred 27 months after the metastasis-free survival analysis.1
*Or after bilateral orchiectomy.1
†Overall survival was measured as the interval from randomization to death from any cause.7
XTANDI + GnRH therapy* significantly delayed time to first use of new antineoplastic therapy† in patients with nmCRPC1
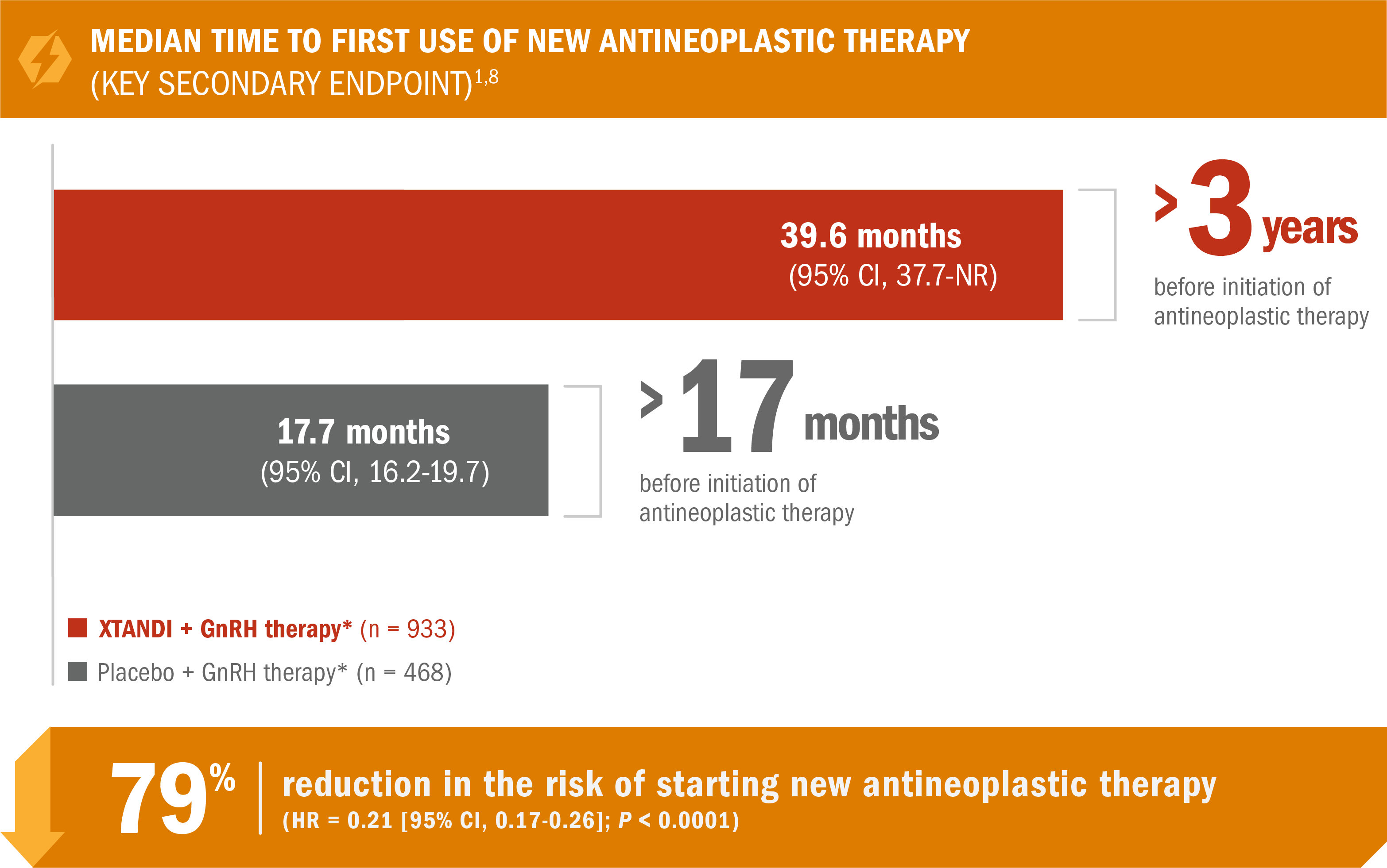
- Number of events: 142 (15%) with XTANDI + GnRH therapy* vs 226 (48%) with placebo + GnRH therapy*2
*Or after bilateral orchiectomy.1
†Defined as the time from randomization to first use of a new antineoplastic therapy for prostate cancer. Subsequent prostate cancer therapies included cytotoxic, hormonal (except GnRH agonist/antagonist), or investigational therapies.5,9
Additional results from PROSPER in patients with nmCRPC8
These PSA data are not reported in product labeling because PSA is not a reliable surrogate for overall survival. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.
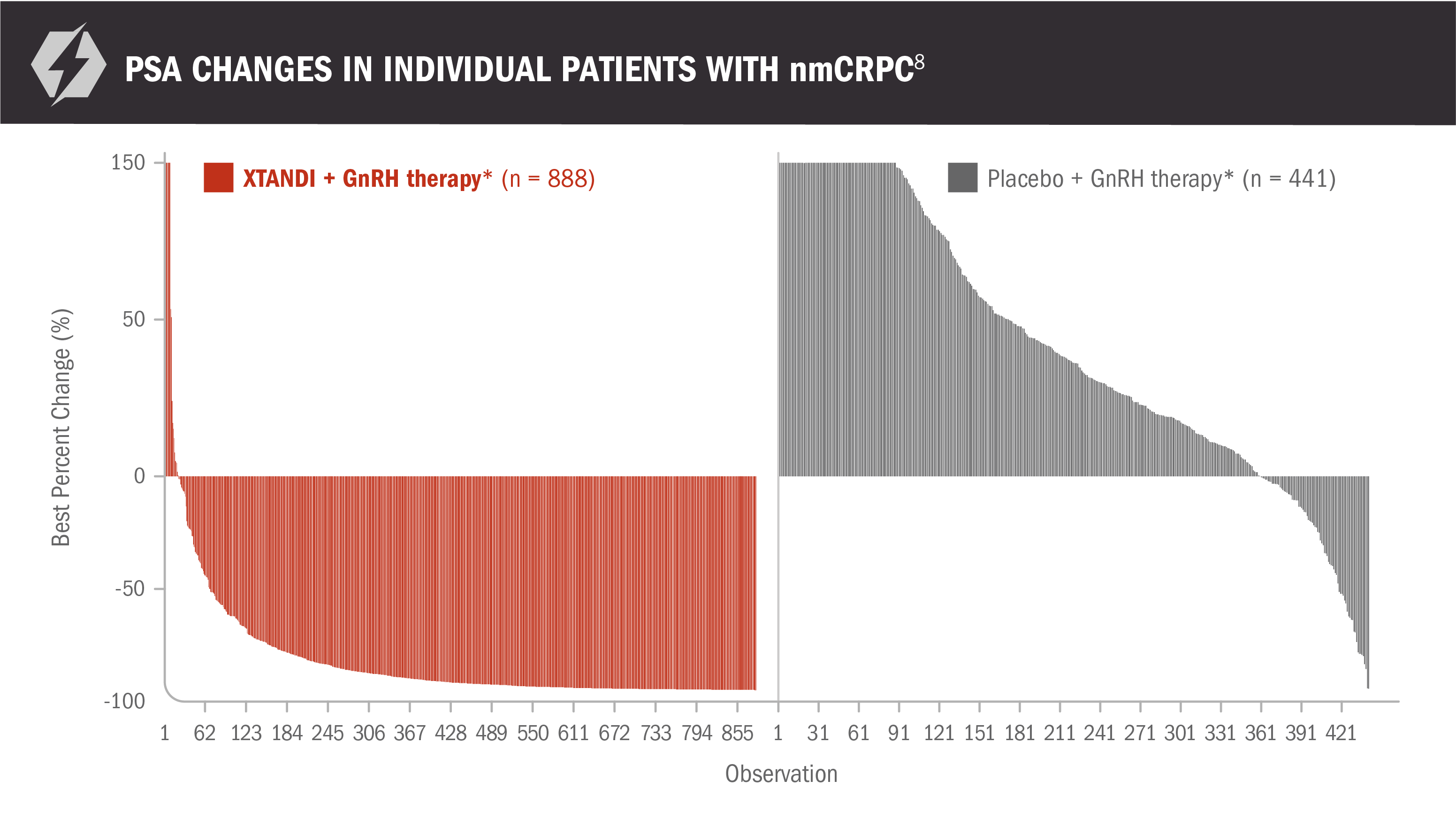
- Reductions in PSA of ≥ 50% were observed in 76% (95% CI, 74%-79%) of patients receiving XTANDI + GnRH therapy* and 2% (95% CI, 1%-4%) of patients receiving placebo + GnRH therapy*8
*Or after bilateral orchiectomy.1
XTANDI in nmCRPC
(PROSPER TRIAL
PUBLICATION)
XTANDI in nmCRPC
(PROSPER TRIAL PUBLICATION)
Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892.
View The PublicationBICR, blinded independent central review; CI, confidence interval; CRPC, castration-resistant prostate cancer; ECOG, Eastern Cooperative Oncology Group; FDA, U.S. Food and Drug Administration; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; mCRPC, metastatic castration-resistant prostate cancer; nmCRPC, nonmetastatic castration-resistant prostate cancer; NR, not reached; PSA, prostate-specific antigen.
References: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025. 2. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. doi:10.1056/NEJMoa1800536. 3. U.S. Food and Drug Administration. Xtandi sNDA 203415/S-014 (PROSPER) approval letter. Published July 13, 2018. Accessed February 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/203415Orig1s014ltr.pdf. 4. U.S. Food and Drug Administration. Xtandi sNDA (203415/S-016) approval letter. Published October 23, 2020. Accessed February 26, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/203415Orig1s016ltr.pdf. 5. Astellas and Pfizer. XTANDI. Data on File. 6. Protocol for: Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. doi:10.1056/NEJMoa1800536. 7. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892. 8. Pfizer. XTANDI. Data on File.
CI, confidence interval; CRPC, castration-resistant prostate cancer; ECOG, Eastern Cooperative Oncology Group; FDA, U.S. Food and Drug Administration; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; ICR, independent central review; mCRPC, metastatic castration-resistant prostate cancer; NR, not reached; NYHA, New York Heart Association; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PSA, prostate-specific antigen; RECIST 1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
References: 1. XTANDI. Package insert. Northbrook, IL: Astellas Pharma US, Inc; 2025. 2. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. doi:10.1056/NEJMoa1405095. 3. Pfizer. XTANDI. Data on File. 4. U.S. Food and Drug Administration. Xtandi sNDA 203415/S-003 (PREVAIL) approval letter. Published September 10, 2014. Accessed February 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/203415Orig1s003ltr.pdf. 5. Protocol for: Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. doi:10.1056/NEJMoa1405095. 6. Astellas and Pfizer. XTANDI. Data on File. 7. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151-154. doi:10.1016/j.eururo.2016.07.032. 8. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. doi:10.1016/S1470-2045(15)00518-5. 9. Supplement to: Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. doi:10.1016/S1470-2045(15)00518-5. 10. Astellas. XTANDI. Data on File. 11. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi:10.1056/NEJMoa1207506. 12. Protocol for: Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi:10.1056/NEJMoa1207506.