View and download useful resources about XTANDI for your office
US Full Prescribing Information for XTANDI,
Including Efficacy, Safety, and Dosing
Get information about XTANDI.
View & DownloadnmCSPC With High-risk BCR Patient Profiles Brochure
Explore profiles of patients with nmCSPC with high-risk BCR who are eligible for XTANDI.
Not actual patients or patient cases; content for illustrative purposes only.
mCSPC Patient Profiles Brochure
Explore profiles of patients with mCSPC who are eligible for XTANDI.
Not actual patients or patient cases; content is for illustrative purposes only.
XTANDI Heritage Flashcard
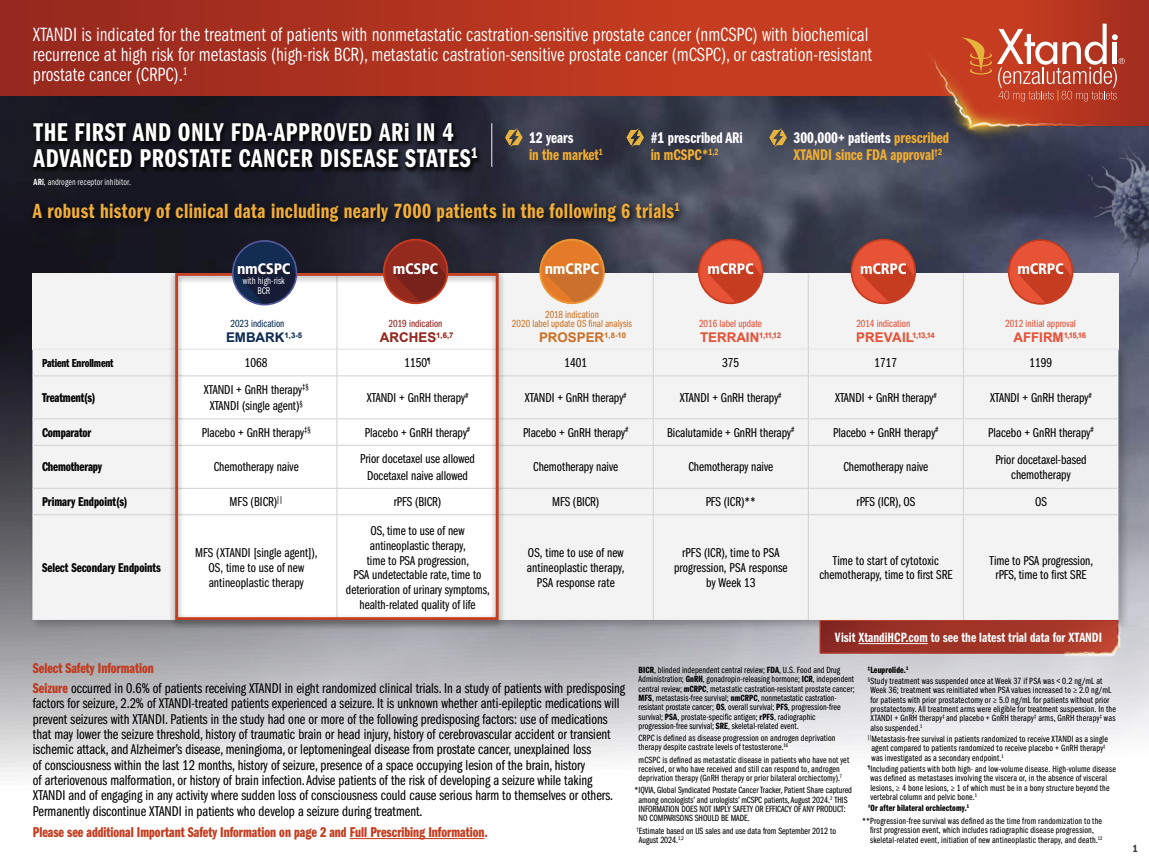
Take a closer look at the details of 6 clinical trials that resulted in XTANDI label updates.
View & DownloadExplore publications that report clinical trial data for XTANDI
Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389(16):1453-1465.
Go To PublicationArmstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986.
Go To PublicationArmstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622.
Go To PublicationHussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
Go To PublicationSternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206.
Go To PublicationPREVAIL
Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151-154.
Go To PublicationTERRAIN
Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163.
Go To PublicationAFFIRM
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197.
Go To PublicationDownload useful resources about XTANDI to share with your patients
Getting Started Brochure
This resource provides patients with information about XTANDI and advanced prostate cancer.
View & DownloadQuestions for Patients to Ask Their Doctor
Patients can use this guide to prepare for their next doctor visit.
View & DownloadSide Effect Self-Management Card
Help patients find ways to self-manage the mild to moderate side effects of XTANDI treatment.
View & DownloadXTANDI Support Solutions® Pamphlet
Patients can learn about XTANDI Support Solutions and get support.
View & DownloadHelp prescribed patients access XTANDI
Option 1: Submit an XTANDI prescription directly to a specialty pharmacy.

Applicable to most insured patients. You may view the most up-to-date list of participants in the XTANDI specialty pharmacy network at XTANDISupportSolutions.com
Option 2: Request to access XTANDI through XTANDI Support Solutions®.*

Applicable to patients without prescription drug benefits
*Program terms and conditions apply.
XTANDI Support Solutions is there to provide:

Astellas Patient Assistance Program (PAP)*
Astellas PAP provides XTANDI at no cost to patients who meet the program eligibility requirements. Eligibility is determined on a patient-specific basis. XTANDI Support Solutions can quickly confirm whether your patient is eligible and answer any questions you may have. Your patients may be eligible for this program if they:
- Are uninsured; a patient is considered uninsured when a patient has no prescription drug insurance†
- Have a verifiable shipping address in the United States
- Have been prescribed XTANDI for an FDA-approved indication
- Meet the program financial eligibility requirements

Information about other assistance options, such as Medicare Part D Extra Help

XTANDI Patient Connect
XTANDI Patient Connect helps connect patients and their caregivers to independent resources and third-party support services that may help them manage their disease and daily life while on treatment. XTANDI Patient Connect resources offer emotional, logistical, and informational support‡
*Subject to meeting eligibility requirements. Void where prohibited by law.
†Other insured patients may be eligible for the program if they meet certain eligibility criteria.
‡Support is provided through third-party organizations that operate independently and are not controlled or endorsed by Astellas. Availability of support and eligibility requirements are determined by these organizations.
Cost and copay support for XTANDI
XTANDI Patient Savings Program
Assistance for Commercially Insured Patients
The XTANDI Patient Savings Program* is for eligible commercially insured patients taking XTANDI tablets. The Program parameters are as follows:

- Patients may pay as little as $0 per prescription
- Patients will be enrolled in the Program for a 12-month period
- There are no income requirements
- There is a maximum copay assistance limit of $7000 per calendar year†
Eligibility restrictions, terms, and conditions apply.
Initiating enrollment on behalf of your patients
HCPs can begin the Program enrollment process on behalf of their patients by following these steps:
STEP 1:
- GO to XTANDIcopayenroll.com
- ENTER your patient's demographic information
STEP 2:
- CONFIRM if you are the HCP or specialty pharmacy enrolling on behalf of your patient
STEP 3:
- ANSWER questions to confirm your patient's eligibility, including their insurance status and place of residence
STEP 4:
- ATTEST that you have shared the terms and conditions for the XTANDI Patient Savings Program with the patient. Confirm that the patient has consented to comply with such terms and conditions
STEP 5:
- PRINT your patient's copay program processing information. If you are the HCP, please send this information to your patient's specialty pharmacy. If you are the specialty pharmacy, please save this information to your patient’s records and process the copay program claim accordingly
- INFORM your patient that they will receive a copy of their copay program details via a mailed letter and email (if provided during enrollment)
*By enrolling in the XTANDI Patient Savings Program ("Program"), the patient acknowledges that they currently meet the eligibility criteria and will comply with the following terms and conditions: The Program is for eligible patients with commercial prescription insurance coverage for XTANDI® (enzalutamide) and is good for use only with a valid prescription for the XTANDI tablet formulation. The Program is not valid for patients whose prescription claims are reimbursed, in whole or in part, by any state or federal government program, including, but not limited to, Medicaid, Medicare, Medigap, Department of Defense (DoD), Veterans Affairs (VA), TRICARE, Puerto Rico Government Insurance, or any state patient or pharmaceutical assistance program. Patients who move from commercial insurance to federal or state health insurance will no longer be eligible, and agree to notify the Program of any such change. Patients agree not to seek reimbursement from any health insurance or third party for all or any part of the benefit received by the patient through the Program. This offer is not conditioned on any past, present, or future purchase of XTANDI. This offer is not transferrable and cannot be combined with any other offer, free trial, prescription savings card, or discount. The full value of the Program benefits is intended to pass entirely to the eligible patient. This offer is not health insurance and is only valid for patients in the 50 United States, Washington DC, Puerto Rico, Guam, and Virgin Islands. This offer is not valid for cash paying patients. This Program is void where prohibited by law. No membership fees. It is illegal to sell, purchase, trade, counterfeit, duplicate, or reproduce, or offer to sell, purchase, trade, counterfeit, duplicate, or reproduce the card. This offer will be accepted only at participating pharmacies. Certain rules and restrictions apply. Astellas reserves the right to revoke, rescind, or amend this offer without notice.
The Program has a maximum copay assistance limit of $7,000 per calendar year. After the annual maximum on copay assistance is reached, patient will be responsible for the remaining out-of-pocket costs for XTANDI. Astellas may reduce or discontinue the copay assistance available under the Program if it determines an enrolled patient is subject to a program offered by a third-party payer or pharmacy benefit manager, or an agent of either, that adjusts patients’ out-of-pocket cost-sharing obligations based on the copay assistance provided by this Program, or excludes the copay assistance provided under this Program from counting towards an enrolled patient’s out-of-pocket cost-sharing obligations (“maximizer” or “accumulator” program). The Program uses advanced logic to identify whether a claim for an enrolled patient is subject to a “maximizer” or “accumulator” program. Unless prohibited by law, Astellas may reduce the cost-sharing assistance available under the Program to a per claim maximum of $25 if it determines a claim for an enrolled patient is subject to a “maximizer” or “accumulator” program.
†Subject to a maximum copay assistance limit of $7,000 per calendar year. Unless prohibited by law, Astellas may reduce the cost-sharing assistance available under the Program to a per claim maximum of $25 if it determines a claim for an enrolled patient is subject to a “maximizer” or “accumulator” program.
Links to patient support organizations
The organizations listed here offer a variety of services that include support, information, and advice to help your patients learn about living with prostate cancer.
Astellas and Pfizer are not affiliated with, do not own or control, and are not responsible for the content or services contained on the websites. The information provided by Astellas and Pfizer is for informational purposes only and is not meant to replace a doctor’s or nurse’s advice.
PATIENTS GET
STARTED
ON XTANDI
GET STARTED ON XTANDI

Or call 1-855-8XTANDI
(1-855-898-2634)
Monday – Friday, 8 AM – 8 PM ET
BCR, biochemical recurrence; GnRH, gonadotropin-releasing hormone; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; nmCRPC, nonmetastatic castration-resistant prostate cancer; nmCSPC, nonmetastatic castration-sensitive prostate cancer.
FDA, U.S. Food and Drug Administration; HCP, healthcare provider.